Biosimilars
Progress for bevacizumab copy biologicals from Henlius and Innovent
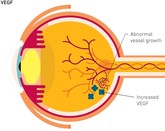
Shanghai Henlius Biotech, Inc (Henlius) reports progress in clinical trials of a bevacizumab copy biological for age-related macular degeneration and colorectal cancer, while Innovent Biologics reports positive findings from a trial of their bevacizumab copy biological in hepatocellular carcinoma.
Data support the interchangeability of EU biosimilars
The first study to comprehensively analyse post-marketing surveillance data for biosimilar monoclonal antibodies demonstrates comparable efficacy, safety and immunogenicity with the originator products [1].
FDA approves adalimumab biosimilar Cyltezo as interchangeable
The US Food and Drug Administration (FDA) has approved the first interchangeable adalimumab biosimilar. This is the second interchangeable biosimilar to gain FDA approval in the US and follows the landmark decision to approve an interchangeable insulin glargine biosimilar in July 2021 [1].
Patent litigation insights on barriers to US biosimilar market growth
High prices of biological drugs have placed substantial strain on the US healthcare system. To help address this problem, Congress passed the Biologics Price Competition and Innovation Act of 2009 (BPCI Act) as part of the Affordable Care Act, which created an abbreviated approval pathway for biosimilars – versions of ‘originator’ biological drugs made by different manufacturers. To resolve disputes over whether a biosimilar manufacturer would infringe patents on the originator biological by entering the market, the BPCI Act included a multi-step litigation process, which starts when the biosimilar manufacturer submits its abbreviated biological license application to the US Food and Drug Administration (FDA). However, a decade after its passage, the BPCI Act has spurred only limited competition [1].
Bio-Thera and Samsung Bioepis start clinical trials for ustekinumab biosimilars
China’s Bio-Thera Solutions and South Korea’s Samsung Bioepis have begun phase III clinical trials for biosimilars to Janssen’s blockbuster anti-inflammatory drug Stelara (ustekinumab).
Argentinian gastroenterologist groups issued position statement on biosimilars use
Experts from the steering committees of the Argentinian Society of Gastroenterology (SAGE), the Argentinian Federation of Gastroenterology (FAGE) and the Argentinian Group of Crohn's Disease and Ulcerative Colitis (GADECCU) have issued a joint position regarding the practical conditions for the clinical use of biosimilars indicated for inflammatory bowel diseases (IBDs), by discussing the most notable aspects related to the definition of a biosimilar drug, bio-similarity, non-comparable biological drugs or ‘intended copies’, approval requirements, extrapolation of indications, interchangeability, automatic substitution, non-medical switching, nomenclature, clinical standards regarding safety and efficacy, implementation of an efficient and appropriate pharmacovigilance system and the potential economic impact on the healthcare system [1].
Canada approves adalimumab and bevacizumab biosimilars
Canada’s drug regulator, Health Canada, has approved the bevacizumab biosimilar Bambevi and the adalimumab biosimilar Abrilada (PF-06410293).
Research, development and public production of pharmaceuticals in Argentina
Under the current research and development model, the pharmaceutical industry has switched its focus towards the therapeutic areas that offer the greatest commercial benefit, which are often not aligned with public health needs. Consequently, it has ceased to be the great innovative industry that it had been during the last century.
Delayed biosimilars market entry costs US billions
Delayed adalimumab biosimilar entry to the US market is estimated to have cost Medicare over US$2.19 billion between 2016 and 2019, a study published in Clinical Pharmacology and Therapeutics has revealed.
Competition from biosimilars drives price reductions for biologicals in France
The US has experienced a policy debate as to whether competition from biosimilars is the best strategy for achieving price reductions for biologicals or, rather, whether direct price regulation after loss of patent exclusivity would be more effective. In order to investigate this issue, authors from the US and France combined quantitative and case study methods to examine in detail the interaction between market and administrative mechanisms to reduce biologicals’ prices in France. In their article they present comprehensive data on market shares and prices for three major biologicals and their 11 competing biosimilars between 2004 and 2020 [1].