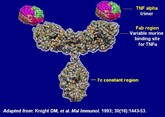
Biosimilars
Biosimilar infliximab receives approval in Japan and Turkey
South Korean biotechnology company Celltrion announced on 4 and 16 July 2014 that the company had received marketing approval for its biosimilar Remsima (infliximab) in Japan and Turkey, respectively.
Biosimilar trastuzumab similar to Herceptin in non-clinical study
Comparative non-clinical assessments of the proposed trastuzumab biosimilar PF-05280014 and the originator product (Herceptin) sourced in the US and in Europe showed similar structural properties, tumour cell growth inhibition properties and pharmacokinetic profiles, as well as safety profiles [1].
FDA accepts biosimilar filgrastim application
Sandoz, the generic drug division of Swiss drug giant Novartis, announced on 24 July 2014 that the US Food and Drug Administration (FDA) had accepted its application for approval of the company’s biosimilar filgrastim product.
Biosimilars of darbepoetin alfa
Last update: 20 November 2020
Darbepoetin alfa is a synthetic form of erythropoietin. It stimulates erythropoiesis (increases red blood cell levels) and is used to treat anaemia, commonly associated with chronic renal failure and cancer chemotherapy.
Phase I studies of infliximab and rituximab biosimilars demonstrate pharmacokinetic similarity
Results of phase I trials of pharma giant Pfizer’s biosimilar infliximab and rituximab candidates have demonstrated similar pharmacokinetic properties compared to the originator products [1, 2].
32 organizations agree biosimilars should have same names
On 1 July 2014, 32 organizations signed a letter calling on the US Food and Drug Administration (FDA) to require biologicals and biosimilars to have the same International Nonproprietary Name (INN).
EMA approves biosimilar insulin
The European Medicines Agency’s (EMA’s) Committee for Medicinal Products for Human Use (CHMP) announced on 27 June 2014 that it had recommended granting of marketing authorization for a biosimilar insulin glargine product (LY2963016).
Biosimilar etanercept demonstrates equivalent efficacy
A phase III trial comparing Hanwha Chemical Corporation (Hanwha)’s biosimilar etanercept, HD203, with Enbrel (etanercept) has demonstrated equivalent efficacy [1].
ECCO position statement on biosimilars
The European Crohn’s and Colitis Organisation (ECCO) is a non-profit association with the aim of improving the care of patients with inflammatory bowel disease (IBD) in Europe. The association currently includes 2,519 individual experts, 33 country members and 17 corporate members.
Biosimilar infliximab comparable to Remicade
Results from a phase III trial have demonstrated the comparability of US-based Epirus Biopharmaceuticals (Epirus) biosimilar (BOW015) to Remicade for the treatment of rheumatoid arthritis.