Biosimilars
FDA receives application for monoclonal antibody biosimilar
South Korean biotechnology company Celltrion announced on 11 August 2014 that the company had, on 8 August 2014, completed the filing procedure to obtain US Food and Drug Administration (FDA) approval for its infliximab biosimilar.
FDA grants tentative approval for insulin treatment
On 18 August 2014, the US Food and Drug Administration (FDA) granted tentative approval for a new insulin glargine product (LY2963016).
ECCO survey highlights lack of confidence in biosimilar mAbs
In a presentation at the EuropaBio and the Alliance for Safe Biologic Medicines (ASBM) roundtable on naming, transparency and traceability for biosimilars [1], held on 18 March 2014 in Brussels, Belgium, Dr Alessandro Armuzzi presented results of a survey of European Crohn’s and Colitis Organisation (ECCO) members [2]. The results of the survey highlight the lack of confidence ECCO members have in biosimilars and the need for continued education.
More biosimilars collaborations on the cards
Collaborations for biosimilars are still in vogue, with the latest companies making deals being PlantForm Corporation (PlantForm) with PharmaPraxis and Oncobiologics and Laboratorios Liomont.
WHO proposal offers clarity for biosimilar nomenclature
Following requests from drug regulatory authorities worldwide, the World Health Organization (WHO) has released a draft Biological Qualifier (BQ) proposal on which to base a globally recognized naming scheme for biological products, including biosimilars. According to the proposal, a four-letter code – the BQ – would be added after the INN.
Biosimilars in the treatment of chemotherapy-induced anaemia
A study of the use of epoetin biosimilars in the therapeutic management of anaemia secondary to chemotherapy in haematology and oncology has shown the biosimilars to be effective and well tolerated in the management of chemotherapy-induced anaemia in patients with solid tumours, lymphoma and myeloma [1].
Biosimilars of cetuximab
Last Update: 13 April 2018
Cetuximab is a chimeric (mouse/human) monoclonal antibody. It inhibits epidermal growth factor receptor (EGFR) and is used to treat metastatic colorectal cancer, metastatic non-small cell lung cancer and head and neck cancer.
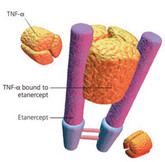
Coherus starts phase III biosimilar etanercept trial
US-based biosimilars developer Coherus BioSciences (Coherus) announced on 23 June 2014 the start of a global phase III trial for a biosimilar version of etanercept.
Biosimilars deals coming thick and fast
Biosimilar collaborations are still the latest fashion, with new deals being made between Cipla and Mabpharm, Strides Arcolab and Oncobiologics, and NeuClone and the Serum Institute.
Switching and extrapolation of subsequent entry biologics in Canada
The regulatory framework for biosimilars in Canada explains how their substitutability and/or interchangeability are governed in the country. Biosimilars, which are known as subsequent entry biologics (SEBs) in Canada, are regulated in line with guidance from the World Health Organization.