Biosimilars
Latin American deal for infliximab biosimilar
US-based Epirus Biopharmaceuticals (Epirus) announced on 14 May 2015 that it had made a deal with biosimilars specialist mAbxience, a subsidiary of Spanish healthcare firm the Chemo Group, for Latin America with respect to the infliximab biosimilar made by Epirus.
GPhA launches Biosimilars Council
As part of ongoing efforts to educate and expand access to biosimilars, industry group, the US Generic Pharmaceutical Association (GPhA), has launched its Biosimilars Council, a division of GPhA.
Baxter and Coherus amend biosimilar etanercept collaboration
US-based biosimilars developer Coherus BioSciences (Coherus) and Baxter International (Baxter) announced on 15 April 2015 that they had amended some of the financial terms of their collaboration agreement for CHS-0214, an etanercept biosimilar candidate.
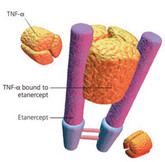
Clinical trials for etanercept biosimilars
Researchers from the Charité – Universitätsmedizin Berlin, Germany, carried out a systematic review into clinical trials for etanercept biosimilars as part of their investigation into biosimilars for the treatment of psoriasis [1].
Australian approval for biosimilar insulin
Australia’s Pharmaceutical Benefits Advisory Committee (PBAC) has given a positive recommendation for the listing of Eli Lilly’s biosimilar insulin glargine, Basaglar in the country’s Pharmaceutical Benefits Scheme (PBS). Basaglar is the first biosimilar insulin to be approved in Australia, providing an alternative, affordable treatment option for patients suffering from diabetes.
Variation in recombinant streptokinase
A study of blood clot dissolving drug streptokinase has highlighted the mantra of ‘the process is the product’ in the case of biologicals [1].
Clinical trials for adalimumab biosimilars
As part of their investigation into biosimilars for the treatment of psoriasis, researchers from the Charité – Universitätsmedizin Berlin, Germany, carried out a systematic review into clinical trials for adalimumab biosimilars [1].
Iran approves its first rituximab biogeneric
In May 2015, Iran’s National Regulatory Authority, the Food and Drug Organization (FDO), approved its first rituximab biogeneric (Zytux). The medicine received its marketing authorization based on the previously published national guideline for marketing of biogenerics in Iran [1].
Biosimilar filgrastim highly similar to originator filgrastim
A study comparing Sandoz’s filgrastim biosimilar (Zarzio) with originator filgrastim (Neupogen) has shown that they are highly similar in terms of their structure and function [1].
Clinical trials of biosimilars for psoriasis treatment
Tumour necrosis factor-alpha (TNF-alpha) is a cytokine, or protein, that prompts the body to create inflammation. In psoriasis and psoriatic arthritis, there is excess production of TNF-alpha in the skin or joints. Therefore, drugs that block TNF-alpha, such as Enbrel (etanercept), Humira (adalimumab), Remicade (infliximab) and Stelara (ustekinumab), can be used to treat psoriasis and psoriatic arthritis.