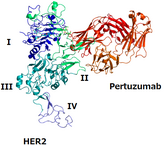
On 13 November 2025, the US Food and Drug Administration (FDA) approved Shanghai Henlius Biologics’ Poherdy (pertuzumab-dpzb) as an interchangeable biosimilar to Genentech’s Perjeta. Additionally,...
More > 0
0

On 13 November 2025, the US Food and Drug Administration (FDA) approved Shanghai Henlius Biologics’ Poherdy (pertuzumab-dpzb) as an interchangeable biosimilar to Genentech’s Perjeta. Additionally,...
More >