A recent report has highlighted how despite the impact of the COVID-19 pandemic in 2020, the volume of biosimilar prescribing has generated a record high in savings from biosimilar competition.
Savings from biosimilars reached an all-time high in 2021
Home/Reports
|
Posted 11/03/2022
 0
Post your comment
0
Post your comment

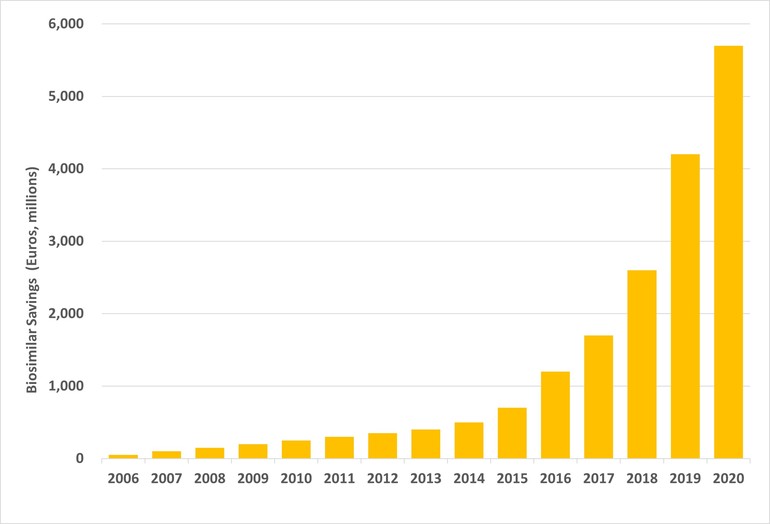
The report, which was carried out by data analysis firm IQVIA at the request of the European Commission, found that list price savings (excluding confidential rebates and discounts) accounted for €5.7 billion in savings versus the pre-biosimilar cost of the originator in 2020, see Figure 1.
Figure 1: List price savings from biosimilar competition
Source: IQVIA [1].
The COVID-19 pandemic has resulted in increasing government debt, which could lead to a focus on easily accessible savings rather than on long-term savings. Due to this, IQVIA believes that ‘ensuring biosimilar competition takes place in a timely manner could be at risk, taking second place to driving uptake. However, a short-term perspective will result in an unsustainable market in a time where system resilience is increasingly important’.
The report concludes that, although savings are naturally higher in countries that have a high use of biologicals, even countries with low usage have seen reductions of 1.5%‒2.5% on their total drug spend.
Conflict of interest
The authors of the report [1] did not provide any conflict-of-interest statement.
Editor’s comment
Readers interested to learn more about savings from biosimilar competition are invited to visit www.gabi-journal.net to view the following manuscript published in GaBI Journal:
Saving money in the European healthcare systems with biosimilars
GaBI Journal is indexed in Embase, Scopus, Emerging Sources Citation Index and more.
Readers interested in contributing a research or perspective paper to GaBI Journal – an independent, peer reviewed academic journal – please send us your submission here.
Related articles
Preparing for future biosimilar opportunities
How biosimilar competition in Europe is changing
Developing access to biologicals remains challenging
Impact of the COVID-19 pandemic on biologicals
Impact of biosimilar competition in Europe in 2021
Reference
1. Troein P, Newton M, Scott K, et al. The impact of biosimilar competition in Europe: December 2021. IQVIA.
| LATIN AMERICAN FORUM The new section of the ‘Latin American Forum’ on GaBI has been launched. The objective of this new section is to provide you with all the latest news and updates on developments of generic and biosimilar medicines in Latin America in Spanish. View this week’s headline article: Nomenclatura de biológicos y biosimilares en Argentina Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative. LATIN AMERICAN FORUM Se ha lanzado la nueva sección del ‘Foro Latinoamericano’ sobre GaBI. El objetivo de esta nueva sección es brindarle las últimas noticias y actualizaciones sobre desarrollos de medicamentos genéricos y biosimilares en América Latina en español. Vea el artículo principal de esta semana: Nomenclatura de biológicos y biosimilares en Argentina !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa. |
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2022 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment