According to a review by Machado et al., across seven Latin American nations, biosimilar approval patterns diverge from Canada, Europe, and the US. Anti-anaemic and diabetes treatments are notably lacking approvals, while Brazil emerges as a leader in biosimilar authorization [1].
Follow-on biological/ biosimilar approvals in Latin America by therapeutic class
Home/Reports
|
Posted 03/04/2024
 0
Post your comment
0
Post your comment

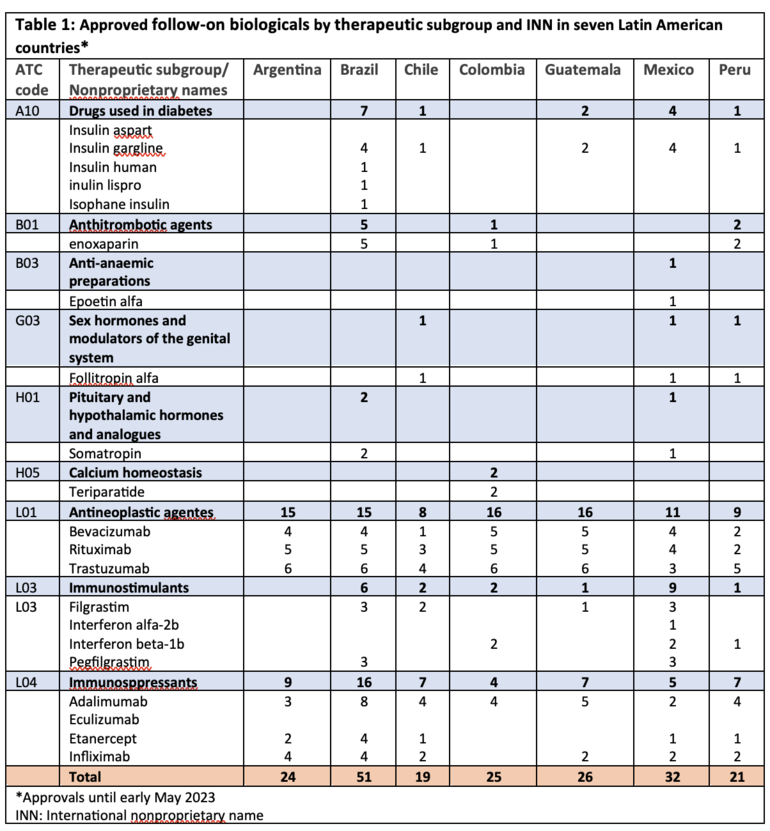
The approval landscape of biosimilars in various therapeutic classes across seven Latin American countries show some differences compared to Canada, Europe and the US.
Table 1 presents the number of biosimilars approved by regulatory authority according to the Anatomical Therapeutic Chemical (ATC) classification and the international nonproprietary name.
In the class of anti-anaemic preparations, only epoetin alfa has secured approval in Mexico. Epoetin lambda, epoetin zeta, and erythropoietin did not receive approval in any of the seven Latin American countries, while Europe and US have approved five and one anti-anaemic preparations, respectively.
Both Argentina and Colombia lack approved biosimilars for medications addressing diabetes, a prevalent lifestyle disease impacting a significant population.
The approval of somatropin and teriparatide biosimilars has only occurred in Brazil and Colombia, respectively, while Canada and Europe have approved 2 and 5 teriparatide biosimilars, respectively.
Additionally, there is an absence of approval for ophthalmological ranibizumab biosimilar in the seven Latin American countries, while Canada, Europe and the US have approved 1, 3 and 2, respectively. Two ranibizumab biosimilars (Byooviz (ranibizumab-runa) and Cimerli (ranibizumab-eqrn)) received interchangeable designation in the US.
By May 2023, Brazil has 52 registered biosimilar medicines and approximately 30 products awaiting analysis or already being analysed by the Brazilian Health Surveillance Agency (Agência Nacional de Vigilância Sanitária, ANVISA) [2]. [2]. This indicates that Brazilian biosimilar approvals have surpassed those of the US Food and Drug Administration, which has approved 47 biosimilars by March 2024 [3], placing Brazil in second position globally for biosimilar approvals, behind only the European Medicines Agency.
Related articles
Biosimilar terminology: insights from seven Latin American countries
First approvals of similar biotherapeutics in seven Latin American countries
Follow-on biological/biosimilar approvals landscape in Latin America
|
LATIN AMERICAN FORUM View the latest headline article: Cuestionando la necesidad de evaluaciones de sensibilidad étnica para anticuerpos monoclonales biosimilares Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
FORO LATINOAMERICANO Ver el último artículo de cabecera: Cuestionando la necesidad de evaluaciones de sensibilidad étnica para anticuerpos monoclonales biosimilares !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa. |
References
1. Machado FLDS, Cañás M, Doubova SV, Urtasun MA, Marín GH, Osorio-de-Castro CGS, et al. Biosimilars approvals by thirteen regulatory authorities: A cross-national comparison. Regul Toxicol Pharmacol. 2023 Sep 1;144:105485. doi: 10.1016/j.yrtph.2023.105485.
2. Cestari de Oliveira SH. Follow-on biologicals/biosimilars approved in Brazil: May 2024 update. Generics Biosimilars Initiative Journal. (GaBI Journal). 2023;12(2):67-72. doi:10.5639/gabij.2023.1202.012
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2024 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment