A new analysis carried out by KFF, a non-profit organization that provides independent information on national health issues, has found that a relatively small share of drugs without generic or biosimilar competitors, accounted for a disproportionate share of prescription drug spending in the US Medicare* system in 2019 [1].
A small number of drugs account for most of Medicare spending
Home/Reports
|
Posted 04/06/2021
 0
Post your comment
0
Post your comment
The analysis follows proposals in the US to lower drug prices. The House, at its 116th Congress, passed legislation (H.R.3) to allow the federal government to negotiate drug prices for up to 250 brand-name drugs lacking generic or biosimilar competition with the highest net spending in Medicare Part D. While the Trump administration issued a final rule to establish a model through the CMS Innovation Center that would base Medicare’s payment for the 50 highest-spending Part B drugs.
In fact, for biosimilars this is perhaps not surprising when considering that biosimilars currently make up only 2.3% of the US biologicals marketplace. Presently, 90% of global biosimilars sales take place in Europe, despite 60% of overall biologicals sales occurring in the US [2].
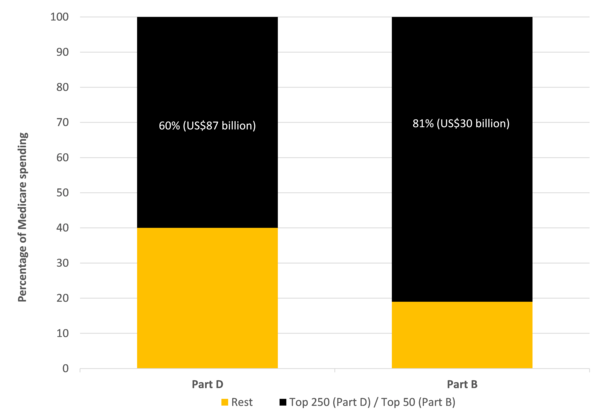
In 2019, the net total Medicare Part D** spending on the 3,500 drugs covered by the scheme was US$145 billion. The KFF analysis found that the 250 top-selling drugs (or 7% of total drugs) with only one manufacturer and no generic or biosimilar medicines competition accounted for approximately US$87 billion or 60% of total drug spending, see Table 1.
For Medicare Part B, the total spending on the 588 drugs covered by the scheme in 2019 was US$37 billion. The KFF analysis found that the 50 top-selling drugs (or 8.5% of total drugs) accounted for approximately US$37 billion or 80% of total drug spending, see Figure 1.
Figure 1: Share of Medicare spending
The findings suggest that focusing on reducing prices for a limited number of high-cost drugs could achieve significant savings.
In the following series of two articles the details of drug spending for Medicare Part D and Part B are discussed.
*Medicare is a national social insurance programme, administered by the US federal government since 1966. It provides health insurance for Americans aged 65 and older who have worked and paid into the system, as well as to younger people with disabilities.
**Medicare Part D is an optional programme to help Medicare beneficiaries pay for their prescription drugs.
#Medicare Part B pays for prescription drugs administered by physicians and other providers in outpatient settings for conditions such as cancer and rheumatoid arthritis.
Related articles
A few drugs account for most of Medicare Part B spending
A few drugs account for most of Medicare Part D spending
| LATIN AMERICAN FORUM – Coming soon! To further enhance the objectives of GaBI in sharing information and knowledge that ensure policies supportive of safe biosimilars use, we are pleased to announce that we will be launching a new section on GaBI Online and GaBI Journal, the ‘Latin American Forum’ (in Spanish) featuring the latest news and updates on research and developments in generic and biosimilar medicines in Latin America. Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
LATIN AMERICAN FORUM – Próximamente! Para fomentar los objetivos de GaBI sobre la difusión de información y conocimiento sobre las políticas de apoyo que garantizan el uso seguro de medicamentos biosimilares, nos complace anunciar el lanzamiento de una nueva sección en GaBI Online y GaBI Journal, el ‘Latin American Forum’ (en español), que presentará las últimas noticias y actualizaciones en investigación y desarrollo sobre medicamentos genéricos y biosimilares en Latinoamérica. Regístrese para recibir el boletín informativo GaBI Latin American Forum. Informe a colegas y amigos sobre esta nueva iniciativa. |
References
1. Cubanski J, Neuman T. Relatively few drugs account for a large share of medicare prescription drug spending. KFF. 19 April 2021.
2. GaBI Online - Generics and Biosimilars Initiative. The sluggish US biosimilars market [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2021 Jun 4]. Available from: www.gabionline.net/Reports/The-sluggish-US-biosimilars-market%20
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2021 Pro Pharma Communications International. All Rights Reserved.
Source: Source: CMS, KFF, US Congress
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment