The European Union (EU) was the first to establish a legal framework for biosimilars back in 2003 [1]. Since the European Medicines Agency (EMA) approved its first biosimilars in 2006 the agency has issued new guidelines and updated its existing guidelines based on new evidence and rapid advances in analytical sciences [2].
Biosimilars applications reviewed in the EU
Home/Reports
|
Posted 06/01/2017
 0
Post your comment
0
Post your comment

In her presentation [3] at the 14th Annual Biosimilar Medicines Group Conference, Dr Martina Weise, Head of the Licensing Division at the Federal Institute for Drugs and Medical Devices (BfArM), discussed the biosimilars applications reviewed by EMA.
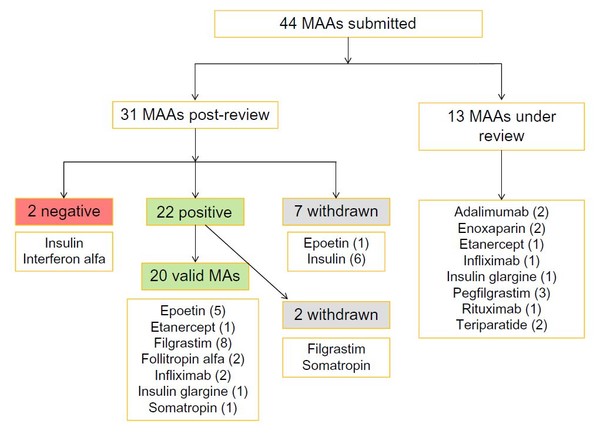
Since EMA started reviewing applications for biosimilars, and up to April 2016, the agency had received 44 marketing authorization applications (MAAs). Of these, 13 were still under review and seven had been withdrawn (six for insulin biosimilars and one for an epoetin biosimilar). Up to April 2016, the agency had given a positive opinion for 22 MAAs, of which two were later withdrawn (filgrastim and somatropin), and a negative opinion for two MAAs (interferon alfa and insulin), see Figure 1.
Figure 1: Biosimilars review by EMA (until April 2016)
EMA: European Medicines Agency; MAAs: marketing authorization applications.
In May 2016, the infliximab biosimilar Flixabi received approval in the EU [1]. In November 2016, the EMA’s Committee for Medicinal Products for Human Use (CHMP) gave a positive opinion for the teriparatide biosimilars, Terrosa and Movymia, and for the insulin glargine biosimilar Lusduna [4].
Disclaimer
The author of the presentation [3] declared that the views and opinions expressed in the presentation were personal views, and may not be understood or quoted as being made on behalf of the CHMP or reflecting the position of the CHMP or any other EMA Committee or Working Party.
Editor’s comment
Readers interested in contributing a research paper in a similar area to GaBI Journal – an independent, peer reviewed academic journal – please send us your submission here.
If you would like to receive a PDF copy* of the Weise presentation [1], please send us an email.
*For profit organizations subjected to a fee
Related articles
Extrapolation and pharmacovigilance for biosimilars
Tailored and reduced clinical programme for biosimilars
Changes in quality data and non-clinical studies for biosimilars
Changes in requirements for reference products for biosimilars
Demonstrating biosimilarity in the EU
References
1. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Jan 6]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Europe
2. GaBI Online - Generics and Biosimilars Initiative. EU guidelines for biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Jan 6]. Available from: www.gabionline.net/Guidelines/EU-guidelines-for-biosimilars
3. Weise M. Evolving landscape on data requirements to demonstrate biosimilarity – the EU perspective. 14th Annual Biosimilar Medicines Group Conference. 28–29 April 2016; London, UK.
4. GaBI Online - Generics and Biosimilars Initiative. EMA approves biosimilars of insulin glargine and teriparatide [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Jan 6]. Available from: www.gabionline.net/Biosimilars/News/EMA-approves-biosimilars-of-insulin-glargine-and-teriparatide
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2017 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment