China is emerging as a global biotech leader, attracting significant investment and partnerships from multinational pharmaceutical companies. Its innovative therapies and biosimilars are gaining international traction, reshaping the industry despite ongoing challenges.
Biosimilars thrive as China’s biotech industry gains momentum
Home/Pharma News
|
Posted 21/01/2025
 0
Post your comment
0
Post your comment

In 2024, China solidified its position as a global hub for biotech innovation, drawing significant attention and investment from multinational pharmaceutical companies.
By early 2022, over 60 pharmaceutical companies were involved in the development of copy biological products [1]. As of September 2023, major international pharmaceutical companies in biosimilar development, including Amgen, Celltrion, Mylan, and Samsung Bioepis, have submitted 17 applications for biosimilars in China. These applications largely target antibody-based biosimilars such as adalimumab, bevacizumab, rituximab, and trastuzumab [2].
Major drugmakers like AbbVie, Bristol-Myers Squibb, Roche, Bayer, and Eli Lilly are deepening their engagement in China, hosting partnering events, and establishing incubators to collaborate with local biotech startups. Pfizer has also pledged to invest US$1 billion in China over the next five years, underscoring the country’s growing role in the biotech sector.
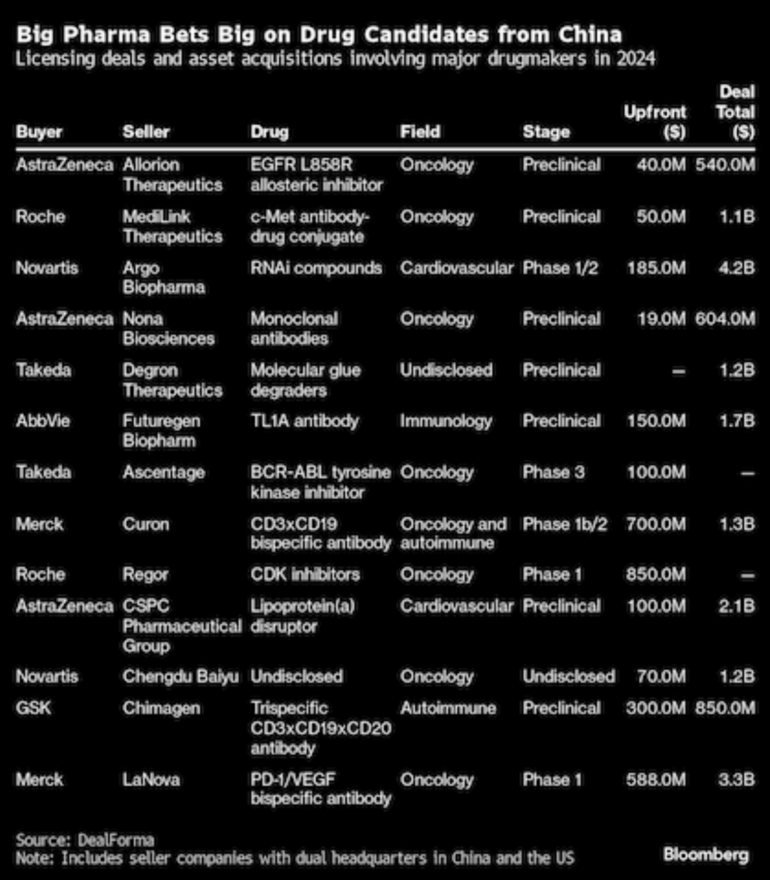
According to DealForma, at least US$3.15 billion was spent in 2024 on licensing or acquiring molecules from Chinese biotech firms. Over the past six years, China’s share of global asset licensing has tripled, reaching 12% of overall deals in 2023–2024. Table 1 presents the licensing deals and assets acquisitions in 2024.
Table 1: Licensing deals and asset acquisitions for drug candidates in China 2024
China’s biotech industry, historically known for generic drugs and outsourcing, is now a hotspot for cutting-edge drug discovery. Government initiatives like ‘Made in China 2025’ have accelerated this transformation by promoting indigenous innovation and aligning regulatory standards with global practices.
This growth is fuelled by the availability of novel therapies at competitive prices, making China an attractive destination for multinational companies seeking to bolster their pipelines amid impending patent expirations.
Despite these advancements, challenges persist. Only five Chinese-developed drugs have been approved by the US Food and Drug Administration (FDA), three of them in 2023. Among these, Brukinsa, a treatment for blood cancer, and Carvykti, a cell therapy used to treat multiple myeloma, have outperformed competitors in clinical efficacy or sales. With limited global track records, questions remain about whether early trial successes in China can be replicated in larger, global studies.
However, several China-produced biosimilars have made inroads in Canada, Europe, and the US. These include:
1. Bio-Thera Solutions’ tocilizumab biosimilar: Tofidence approved in the EU on 20 June 2024; Tofidence (tocilizumab-bavi) approved by the FDA on 29 September 2023. The product is being marketed by Biogen.
2. Bio-Thera Solutions’ bevacizumab biosimilar: Avzivi, approved in the EU on 26 July 2024; Avzivi (bevacizumab-tnjn) approved by the FDA on 6 December 2023. The product is being marketed by Sandoz.
3. Henlius’ trastuzumab biosimilar: Zercepac, approved in the EU on 27 July 2020; Hercessi (trastuzumab-strf) approved by the FDA on 29 April 2024, Adheroza, approved by Health Canada on 22 August 2024. The product is being marketed by Accord.
Other major Chinese biosimilar companies active in foreign markets include Boan Biotech, Innovent Biologics, and Qilu Pharmaceuticals, all with upcoming biosimilars pending for EMA or FDA approval.
Multinational drugmakers see licensing deals with Chinese biotech firms as a lower-risk pathway to accessing innovative therapies. As competition from Chinese biotech intensifies, the global pharmaceutical industry is undergoing a significant shift, with China poised to play a central role in shaping its future.
The continued growth of China’s biotech industry benefits the ongoing development of innovative and biosimilar medicines.
Editor’s comment
European Medicines Agency regulatory requirements ensure the same high standards of quality, safety and efficacy for biosimilars as for originator biologicals, and also include a rigorous comparability exercise with the reference product but they are not universally accepted by regulatory bodies outside of the European Union (EU). It should be noted that copy biologicals approved in China might not have been authorized if they had been subjected to the strict regulatory processes required for approval of biosimilars in the EU.
Related articles
Biosimilars approved in the US
Biosimilars approved in Canada
Biosimilars approved in Europe
|
LATIN AMERICAN FORUM View the latest headline article: Estudios comparativos de eficacia: ¿dónde estamos ahora? Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
FORO LATINOAMERICANO Ver el último artículo de cabecera: Estudios comparativos de eficacia: ¿dónde estamos ahora? !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa. |
References
1. GaBI Online - Generics and Biosimilars Initiative. Pharmaceutical companies in China manufacturing copy biologicals [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2025 Jan 21]. Available from: www.gabionline.net/reports/pharmaceutical-companies-in-china-manufacturing-copy-biologicals
2. GaBI Online - Generics and Biosimilars Initiative. International biosimilars players expanding their presence in China [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2025 Jan 21]. Available from: www.gabionline.net/biosimilars/general/international-biosimilars-players-expanding-their-presence-in-china
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2025 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
Formycon signs new aflibercept biosimilar pacts and launches ranivisio in Europe

Home/Pharma News Posted 13/11/2025
Bio-Thera and Stada expand biosimilars alliance to include tocilizumab

Home/Pharma News Posted 20/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment