A study conducted by Welch J et al. assessed the measurement, evaluation and potential for high mannose glycans to impact the pharmacokinetic (PK) profile of biosimilar monoclonal antibodies (mAbs), based on data submitted in 21 applications reviewed and approved by the US Food and Drug Administration (FDA). They also analysed reproducibility data of the glycan values for a subset of programmes within a reference product class. This reproducibility assessment enabled a broader cross-programme comparison of whether observed differences in high mannose between a proposed biosimilar and its reference product translate into PK differences.
High mannose glycans in biosimilars and their pharmacokinetic impact
Biosimilars/Research
|
Posted 19/05/2023
 0
Post your comment
0
Post your comment
The study found that fluorescence-based hydrophilic interaction liquid chromatography is the methodology employed by most applicants to detect and compare glycans. The characterization of the selected glycans of common IgG1 reference product lots analysed indicate that when applicants use the same analytical methods to analyse glycoforms, the results of the analyses are similar across applications. Welch J et al. then proceeded with a cross-programme comparison for potential differences in high mannose glycans to impact the PK profile of mAbs.
They identified seven out of 21 FDA monoclonal antibody 351(k) applications approved (at the time of the study) with high mannose content that did not overlap with the corresponding reference product high mannose content range. The associated PK similarity study results were gathered to put these analytical differences in high mannose content into a clinical context. All PK similarity studies for these programmes were robustly designed and adequately powered to support a sensitive assessment of whether there were meaningful differences in PK between the biosimilar and the reference product.
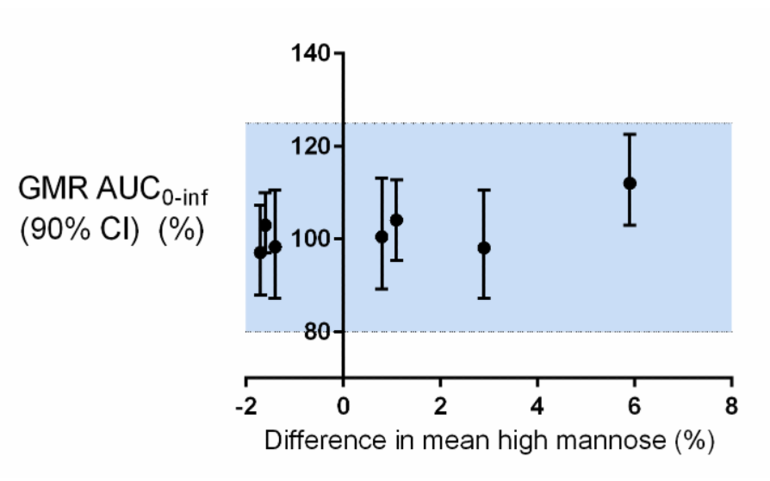
The absolute difference in the mean high mannose measurements between the biosimilar and reference product for these seven programmes ranges from 1.1% to 5.9%, see Figure 1. Of these seven programmes, most biosimilars differ from the reference product in high mannose by less than ± 3%. The GMR of AUC0-inf ranged from 97.1% to 112.0%. Most of the plotted PK results appear clustered around 100% with largely overlapping confidence intervals, suggesting that there is a range of high mannose variation that does not significantly affect exposure as measured by AUC0-inf. One programme with a 5.9% difference in high mannose between the reference product and the biosimilar had a corresponding point estimate and confidence interval for the AUC0-inf comparison that was highest among the seven programmes, see Figure 1. In all cases, the AUC0-inf confidence intervals fell within the pre-defined acceptance criterion for PK similarity (blue shading in Figure 1), confirming that for these programmes, this degree of difference in high mannose does not result in an impact on clinical performance.
Figure 1: Comparison of mannose differences versus PK
PK: pharmacokinetic.
In summary, analytical methods are generally more sensitive than clinical studies at detecting differences between products, should any exist. Despite observed differences in mean high mannose values between the reference product and biosimilar of up to approximately 6%, no clinically meaningful differences in PK similarity were observed for these programmes. This analysis, as well as similar ones, can help establish when certain analytical differences between proposed biosimilars and their reference products will not result in differences in clinical performance.
Conflict of interest
The authors of the research paper [1] declared that there was no conflict of interest.
Abstracted by Dr Nina N Brahme, PhD, MPH and Dr Cristina Ausin, MD; U.S. Food and Drug Administration, Center for Drug Evaluation and Research, Office of New Drugs, Office of Therapeutic Biologics and Biosimilars, Silver Spring, MD, USA.
Editor’s comment
Readers interested to learn more about glycosylated monoclonal antibodies are invited to visit www.gabi-journal.net to view the following manuscript published in GaBI Journal:
GaBI Journal is indexed in Embase, Scopus, Emerging Sources Citation Index and more.
Readers interested in contributing a research or perspective paper to GaBI Journal – an independent, peer reviewed academic journal – please send us your submission here.
GaBI Journal Citation Impact
2.2 – CiteScore 2021 (calculated on 5 May 2022)
2.5 – CiteScoreTracker 2022 (Last updated on 5 April 2023)
Submit a manuscript to GaBI Journal
Related articles
Role of efficacy trials in biosimilar assessments questioned
Afucosylated biosimilars: the path to matching interrelated critical quality attributes
|
LATIN AMERICAN FORUM View the latest headline article: Una actualización sobre la declaración conjunta EMA-HMA sobre la intercambiabilidad de biosimilares Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
FORO LATINOAMERICANO Ver el último artículo de cabecera: Una actualización sobre la declaración conjunta EMA-HMA sobre la intercambiabilidad de biosimilares !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa. |
Reference
1. Welch J, Ausin C, Brahme N, et al. The mannose in the mirror: a reflection on the pharmacokinetic impact of high mannose glycans of monoclonal antibodies in biosimilar development. Clin Pharmacol Ther. 2023;113(5):1003-10.
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2023 Pro Pharma Communications International. All Rights Reserved.
News
FDA approves Poherdy (first interchangeable pertuzumab) and Armlupeg (pegfilgrastim) biosimilars
EMA recommends approval for insulin glargine biosimilar Ondibta and denosumab biosimilar Osqay
General
Samsung Bioepis wins Pyzchiva case; Regeneron patent rulings threaten foreign biosimilars
Chinese biosimilars go global: growth, partnerships, and challenges
What is the future for the US biosimilar interchangeability designation

Biosimilars/Research Posted 05/06/2025
Biosimilar clinical efficacy studies: are they still necessary?

Biosimilars/Research Posted 27/05/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment