Biosimilars – products that are similar to originator biological medicinal products – have had a positive impact on healthcare systems. But it takes up to four years following market approval before biosimilars are accepted by the clinical community and by the people holding the purse strings. Now, a new class of biosimilar –monoclonal antibodies (mAbs) – is set to challenge the system further, writes Professor Andrea Laslop of the Austrian Agency for Health and Food Safety [1].
The future of biosimilar mAbs in Europe
Biosimilars/Research
|
Posted 27/09/2013
 0
Post your comment
0
Post your comment
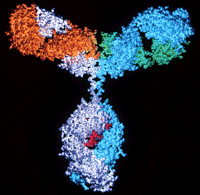
The complexity of a biological product means that no identical copy can be made. The biosimilars that are currently approved in the EU are relatively simple biological compounds, such as human growth hormone, erythropoietin and granulocyte colony-stimulating factor. Monoclonal antibodies are considerably more complicated.
Biosimilar mAbs on the horizon
A biosimlar of the mAb infliximab (Remsima) received approval in Europe on 10 September 2013 [2] and was approved by the Korean FDA in 2012 [3]. There is significant ongoing activity within the biosimilar industry and it is thought that several biosimilar mAbs will receive European marketing approval in the next few years.
There are many obstacles to overcome before biosimilar mAbs can enter daily clinical practice. The demonstration of bioequivalence demands extensive comparability studies at the level of quality characteristics, biological activity and functional characterization. In addition, the collection of post-marketing safety data will be essential to monitor any possible immunogenicity or other rare adverse events.
Saving billions
A significant challenge that could further delay market access is the cost of these biosimilars. Biosimilar mAbs are forecast to cost 10–30% less than originators. This might be considered an insignificant saving in terms of percentage alone, but it has been calculated that this would save between Euros 1.8 and 20.4 billion between 2007 and 2020. Clearly this would have a positive impact on healthcare sustainability.
The clinical community needs to be given sufficient reassurance on the safe use of biosimilars and every effort must be made to avoid unnecessary delay before these valuable products enter the market. Ongoing collection of post-marketing safety data will be of prime importance once these products have been approved.
Editor’s comment
If you are interested in contributing a research paper in a similar area or a Perspective paper on biosimilar mAbs to GaBI Journal, please send us your submission here.
Related articles
More immunogenicity data needed for biosimilar mAbs
Celltrion applies for biosimilar infliximab approval in Japan
Assessment of efficacy and safety of biosimilars in rheumatology
References
1. Laslop A. Biosimilar monoclonal antibodies—challenges and opportunities in Europe. Generics and Biosimilars Initiative Journal (GaBI Journal) 2013;2(3):110-1. doi:10.5639/gabij.2013.0203.034
2. GaBI Online - Generics and Biosimilars Initiative. EC approves first monoclonal antibody biosimilar [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2013 Sep 27]. Available from: www.gabionline.net/Biosimilars/News/EC-approves-first-monoclonal-antibody-biosimilar
3. GaBI Online - Generics and Biosimilars Initiative. Biosimilar monoclonal antibody approved in Korea [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2013 Sep 27]. Available from: www.gabionline.net/Biosimilars/News/Biosimilar-monoclonal-antibody-approved-in-Korea
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2013 Pro Pharma Communications International. All Rights Reserved.
News
FDA approves Poherdy (first interchangeable pertuzumab) and Armlupeg (pegfilgrastim) biosimilars
EMA recommends approval for insulin glargine biosimilar Ondibta and denosumab biosimilar Osqay
General
Samsung Bioepis wins Pyzchiva case; Regeneron patent rulings threaten foreign biosimilars
Chinese biosimilars go global: growth, partnerships, and challenges
What is the future for the US biosimilar interchangeability designation

Biosimilars/Research Posted 05/06/2025
Biosimilar clinical efficacy studies: are they still necessary?

Biosimilars/Research Posted 27/05/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment