The Brazilian pharmaceutical market is the third largest in the Americas region, behind the US and Canada; it ranks first in the Latin American region. Pharmaceutical demand will continue to rise, fuelled by increasing disposable income. Despite this positive outlook, the trade deficit in Brazil grew from US$700 million at the end of the 1980s to a cumulative US$7.13 billion in 2008. In 2008 alone, Brazil imported US$1.4 billion in vaccines, serum and blood products, while exporting US$37 million in medicinal products with low added value [1].
Medicines spending in Brazil
Biosimilars/Research
|
Posted 15/02/2013
 0
Post your comment
0
Post your comment

In Brazil, the Unified Health System (Sistema Único de Saúde, SUS) and the policy of the Ministry of Health (Ministério da Saúde, MS) according to decree GM/MS No. 2.981 of 26 November 2009 grants access to the general population to specialized medicines including some biologicals.
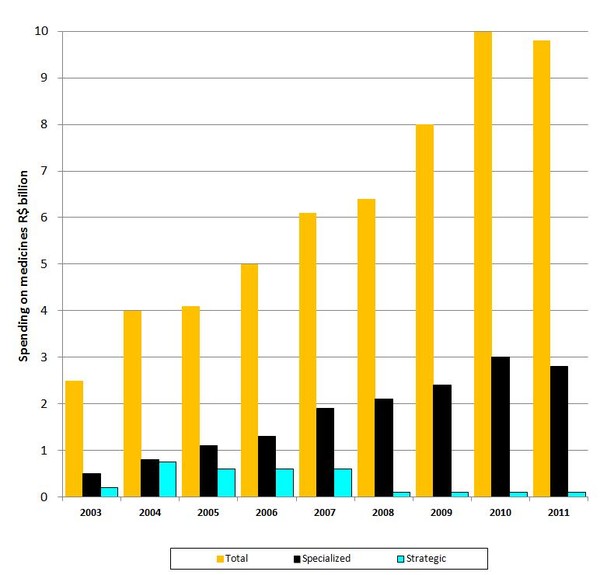
Expenditure on medicines as a whole, and specialized medicines, is constantly increasing in Brazil, see Figure 1. In 2011, 31% of the total amount spent on medicine was for specialized medications including biologicals.
Expenditure on strategic medicines, on the other hand, has reduced. The list of strategic medicines under the SUS is defined according to Decree GM/MS No. 1.284 of 26 May 2010. Strategic drugs are defined as those of higher value, i.e. costing the Brazilian Ministry of Health more than R$10 million per year. Examples of strategic products include filgrastim and somatropin.
Figure 1: Spending on medicines by the Brazilian Ministry of Health
There is therefore an urgent need to combat this increasing expenditure on foreign biologicals. How follow-on biological products as they are called in Brazil, might be produced in Brazil is discussed in the following article.
Editor’s comment
If you are interested in contributing a research article in a similar area to GaBI Journal, please send us your submission here.
Related articles
Prospects for producing follow-on biological products in Brazil
Brazilian guidelines for follow-on biological products
The pharmaceutical market in Brazil
Reference
1. da Silva Madeira L, Borschiver S, Pereira Jr N. Prospects and trends in the Brazilian market for biologically sourced products. J Technol Manag Innov. 2012:7(3);44-56.
Permission granted to reproduce for personal and educational use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
News
EMA recommends approval for teriparatide biosimilar Zandoriah
FDA approves third interchangeable ranibizumab biosimilar Nufymco
General
Samsung Bioepis wins Pyzchiva case; Regeneron patent rulings threaten foreign biosimilars
Chinese biosimilars go global: growth, partnerships, and challenges
What is the future for the US biosimilar interchangeability designation

Biosimilars/Research Posted 05/06/2025
Biosimilar clinical efficacy studies: are they still necessary?

Biosimilars/Research Posted 27/05/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment