In a presentation by Professor JM Walenga and colleagues from the Loyola University Medical Center, Illinois,USA, 'immunogenicity issues faced by biosimilar low molecular weight heparins (LMWHs)' were discussed [1].
Immunogenicity of biosimilar low molecular weight heparins
Biosimilars/Research
|
Posted 22/04/2011
 0
Post your comment
0
Post your comment

Heparin and LMWHs are heterogeneous mixtures of glycosaminoglycans (GAGs), which are used as anticoagulants for the treatment of thrombotic and cardiovascular disorders.
Micro-chemical differences in the heparin molecules affect the biological properties of GAGs, causing differences in the biological activity of the different molecules.
LMWHs readily bind to plasma proteins, such as platelet factor 4 (PF4). When GAGs bind to proteins immunogenic responses can occur, the most common of which is heparin-induced thrombocytopenia (HIT), which causes the development of thrombocytopenia (a low platelet count).
Although symptomatic HIT infrequently occurs in patients treated with LMWHs, anti-heparin/PF4 antibodies (HIT antibodies) develop in up to 25% of treated patients. These antibodies are thought to contribute to the variation in therapeutic response to LMWHs and to the clinical outcome of the patient. The FDA has therefore requested comparative data on immunogenetic responses for biosimilar LMWHs compared to the originator products.
In this study the generation of HIT antibodies and their subtypes was evaluated by enzyme-linked immunosorbent assay (ELISA) in blood samples obtained from clinical studies of different LMWHs.
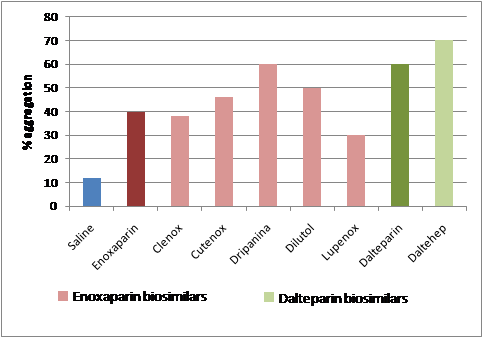
The results showed that in the platelet aggregation HIT assay, the biosimilar versions of enoxaparin all showed different responses to HIT antibodies, some of which were more similar to the branded product than others.
The biosimilar version of dalteparin showed a similar response to HIT antibodies compared to the branded product.
For brand name LMWHs the prevalence of HIT antibodies was found to vary from 12–18%.
The authors concluded that these studies suggest that compositional differences of each LMWH (branded or biosimilar) are determinants of the immunogenic effects of these drugs. They also recommended that the immunogenic profile should be used to define the bioequivalence of LMWHs.
Related articles
EMA proposes more precise definition for biosimilars
Biosimilar low molecular weight heparins in Brazil
Reference
1. Walenga JM, Hoppensteadt D, Cunanan J, et al. Immunogenicity of low molecular weight heparins and their biosimilars. 3rd Workshop on the characterization of heparin products. July 27-28, 2009. USP Headquarters, Rockville, Maryland, USA.
News
FDA approves Poherdy (first interchangeable pertuzumab) and Armlupeg (pegfilgrastim) biosimilars
EMA recommends approval for insulin glargine biosimilar Ondibta and denosumab biosimilar Osqay
General
Samsung Bioepis wins Pyzchiva case; Regeneron patent rulings threaten foreign biosimilars
Chinese biosimilars go global: growth, partnerships, and challenges
What is the future for the US biosimilar interchangeability designation

Biosimilars/Research Posted 05/06/2025
Biosimilar clinical efficacy studies: are they still necessary?

Biosimilars/Research Posted 27/05/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment