This article discusses the issue of immunogenicity with respect to originator biologicals and biosimilars [1].
Immunogenicity of biologicals
Biosimilars/Research
|
Posted 25/05/2012
 0
Post your comment
0
Post your comment
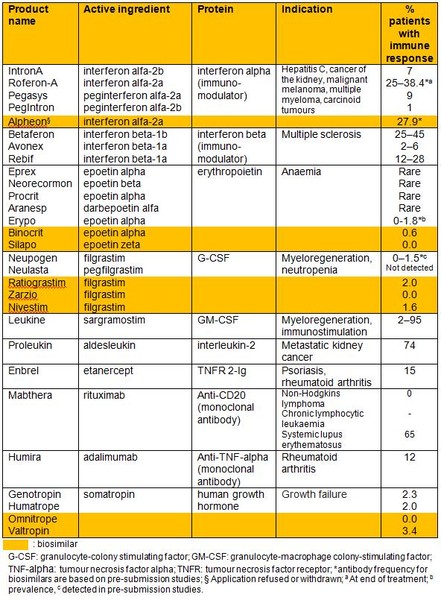
Biotechnology derived products such as coagulation factors, cytokines, enzymes, fusion proteins, hormones and monoclonal antibodies, have proved to be a big success for treating a wide variety of diseases including cystic fibrosis, multiple sclerosis and rheumatoid arthritis. However, despite their obvious benefits therapeutic proteins have the potential to induce an immune response, see Table 1, due either to their foreign nature if of exogenous origin or due to the body recognising the drug as ‘non-self’.
Table 1: Immunogenicity of therapeutics of originator biologicals and biosimilars*
Source: Wadhwa et al. [1]
For a high-resolution copy of Table 1*, please contact us.
*For profit organisations subjected to a fee
Clinical consequences of immunogenicity
Most biologicals induce immune responses, because they are polypeptides or proteins and might therefore be recognised by the immune system as foreign. Immunogenicity is the capability of a specific substance to induce an unwanted immune response that is triggered by more than one single factor. Although in many cases the presence of antibodies may have little clinical consequence, the case of pure red cell aplasia raises concerns about potential clinical consequences of extensive use of biologicals and biosimilars.
Such consequences can be classified as benign, i.e. non life-threatening, and can have effects on efficacy–reducing the clinical response, or on safety. Non-acute consequences can cause delayed-type hypersensitivity/immune complexes or cross-reactivity with an endogenous counterpart, while acute consequences include infusion and anaphylactic reactions.
Factors influencing immunogenicity
Immunogenicity can be influenced by the product itself, but can also be affected by the patient.
Product-related factors include:
- Protein (structure, primary sequence, novel epitopes, glycosylation, oxidation, deamidation)
- Product impurities, formulation, aggregation, degradation
- Properties of the protein, e.g. immunostimulatory, replacement therapy, physiologically important
- Dose, route, frequency of administration and duration of therapy
Patient-related factors include:
- Age
- Gender
- Genetic make-up
- Ethnic sensitivity (IFN-alpha 2a more immunogenic in Chinese compared to Caucasian hepatitis patients 39% vs 14%)
- Immune status
- Disease
The problem with immunogenicity is that it is impossible to predict:
- the incidence of unwanted immunogenicity
- the characteristics of the immune response
- the clinical consequences and significance.
Therefore, such immunogenicity needs to be assessed in appropriate studies.
Regulatory guidance on immunogenicity
In European regulatory guidelines, emphasis has been given to immunogenicity, since any subtle change introduced in the manufacturing process of a biological product can have enormous implications for immunogenicity and EMA already has two guidance documents covering the subject.
Immunogenicity assessment of biotechnology-derived therapeutic proteins EMEA/CHMP/BMWP/14327/2006
Effective date: April 2008
www.emea.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003946.pdf
Immunogenicity assessment of monoclonal antibodies intended forin vivoclinical use EMEA/CHMP/BMWP/86289/2010
End of consultation (deadline for comments): 31 May 2011 (not yet effective)
www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/11/WC500099362.pdf
Immunogenicity of biosimilars
The immunogenicity of the marketed originator product does not influence the need for comparative immunogenicity studies between the originator and biosimilar products. However, if the immunogenicity profiles of marketed and biosimilar products are significantly different, they may be considered dissimilar, making testing for immunogenicity a critical issue when it comes to biosimilars.
Conflict of interest
The author declared that there is no real or apparent conflicts of interest to report. The views expressed by the author in the presentation are her own and do not represent her institution or any boards on which she has served.
Editor’s comment
If you are interested in contributing a research article in a similar area to GaBI Journal, please send us your submission here.
Related articles
Epoetin alfa and pure red cell aplasia
Reference
1. Wadhwa M. Immunogenicity: what do we know and what can we measure? Satellite symposium, 16th Congress of the EAHP; 2011 Mar 30; Vienna, Austria.
News
FDA approves Poherdy (first interchangeable pertuzumab) and Armlupeg (pegfilgrastim) biosimilars
EMA recommends approval for insulin glargine biosimilar Ondibta and denosumab biosimilar Osqay
General
Samsung Bioepis wins Pyzchiva case; Regeneron patent rulings threaten foreign biosimilars
Chinese biosimilars go global: growth, partnerships, and challenges
What is the future for the US biosimilar interchangeability designation

Biosimilars/Research Posted 05/06/2025
Biosimilar clinical efficacy studies: are they still necessary?

Biosimilars/Research Posted 27/05/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment