Since COVID-19, caused by SARS-CoV-2, emerged as a worldwide concern and was declared a pandemic, finding a safe and effective treatment for this disease has been a top priority. Different treatment candidates, including interferon [1], remdesevir [2], tocilizumab [3], and dexamethasone [4] have been investigated in multiple clinical trials.
Efficacy and safety of interferon beta-1a (ReciGen) in COVID-19
Biosimilars/Research
|
Posted 05/03/2021
 0
Post your comment
0
Post your comment
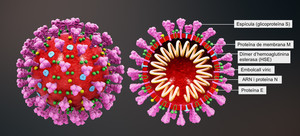
In all viral infections, like corona virus disease including COVID-19, the infected cell relies on immune responses to restrict the transfection of healthy cells by the virus. One of the first cytokines that is produced by infected cells is the type I interferon (interferon beta). Due to the reported efficacy of interferon beta in SARS-CoV [5] and its mechanism of action, different clinical trials using interferon beta were conducted worldwide. Based on the evidence, which supports efficacy of interferon beta-1a in COVID-19, clinical trials with ReciGen (interferon beta-1a, CinnaGen) were designed and conducted in multiple centres in Iran.
In the first clinical trial published by Payandemehr et al. [6], the efficacy and safety of ReciGen were evaluated in 20 patients who had confirmed cases of COVID-19 and were treated by this product in combination with the current local COVID-19 protocol for 5 consecutive days. It was observed that the median (interquartile range) hospitalization duration was 5.0 (3–6) days and 15 (94%) patients reported good general health condition 5 days after discharge without any lasting significant complaints. Likewise, no case of re-admission or death was reported after discharge. These results verified the efficacy of ReciGen to improve the health and reduce the hospitalization time of patients with an acceptable safety profile.
The next study that evaluated the efficacy and safety of ReciGen in COVID-19 patients was published by Davoudi-Monfared et al. [7]. In this study, 92 patients were enrolled and 42 patients received interferon beta-1a along with the current national protocol medications and 39 patients received only the current national protocol medications as a control group and completed the study. Results of the study revealed that treatment with ReciGen significantly reduced the 28-day overall mortality (p = 0.015) compared to the control group and early administration was one of the main factors to increase the efficacy of treatment with ReciGen. Moreover, addition of this product to the treatment protocol, lead to less hospitalization time in the treatment group compared to the control group.
The next study that reported the efficacy of ReciGen was published by Dastan et al. [8]. In this prospective trial, 20 COVID-19 confirmed patients were treated by conventional treatment protocol including ReciGen. The results of the study including lung computed tomography (CT) and chest X-ray illustrated the significant efficacy of ReciGen in combination with conventional treatment in improving the health of patients. In addition, during the 4-day study period, no case of death or significant adverse drug reactions were reported and symptoms of disease including cough, dyspnea and myalgia decreased gradually.
Finally, in a recent article, Baghaei et al. [9] reported the efficacy evaluation of ReciGen in 456 COVID-19 patients (152 interferon beta-1a group and 304 control group) in a randomized, double-arm study. The group treated with interferon beta-1a resulted in a good prognosis and had a lower mortality rate. The interferon beta-1a-treated group had less mortality (at least five times) than the control group, which was significant (p < 0.001) when considering oxygen support and treatment-improved oxygen support.
In conclusion, overall, 588 patients with confirmed COVID-19 were enrolled in these studies. The results verified the lower hospitalization time, improved prognosis and lower mortality rate for patients treated with ReciGen. This was in accordance with the results of other clinical trials which evaluated the efficacy of interferon beta-1a with different doses and routes of administration, such as inhaled nebulised interferon beta-1a [1].
Related articles
Russian approval for non-originator interferon beta-1a
EMA publishes draft biosimilar guideline for interferon beta
| LATIN AMERICAN FORUM – Coming soon! To further enhance the objectives of GaBI in sharing information and knowledge that ensure policies supportive of safe biosimilars use, we are pleased to announce that we will be launching a new section on GaBI Online and GaBI Journal, the ‘Latin American Forum’ (in Spanish) featuring the latest news and updates on research and developments in generic and biosimilar medicines in Latin America. Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
LATIN AMERICAN FORUM – Próximamente! Para fomentar los objetivos de GaBI sobre la difusión de información y conocimiento sobre las políticas de apoyo que garantizan el uso seguro de medicamentos biosimilares, nos complace anunciar el lanzamiento de una nueva sección en GaBI Online y GaBI Journal, el ‘Latin American Forum’ (en español), que presentará las últimas noticias y actualizaciones en investigación y desarrollo sobre medicamentos genéricos y biosimilares en Latinoamérica. Regístrese para recibir el boletín informativo GaBI Latin American Forum. Informe a colegas y amigos sobre esta nueva iniciativa. |
References
1. Monk PD, Marsden RJ, Tear VJ, et al. TN. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med. 2021;9(2):196-206
2. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - Final Report. N Engl J Med. 2020;383(19):1813-26.
3. Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384(1):20-30
4. The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704.
5. Hensley LE, Fritz LE, Jahrling PB, et al. Interferon-β 1a and SARS coronavirus replication. Emerg Infect Dis. 2004;10(2):317-9.
6. Payandemehr P, Azhdarzadeh M, Bahrami-Motlagh H, et al. Interferon beta-1a as a candidate for COVID-19 treatment; an open-label single-arm clinical trial. Front Emerg Med. 2020;4(2S):e51.
7. Davoudi-Monfared E, Rahmani H, Khalili H, et al. A randomized clinical trial of the efficacy and safety of interferon β-1a in treatment of severe COVID-19. Antimicrob Agents Chemother. 2020;64(9):e01061-20.
8. Dastan F, Nadji SA, Saffaei A, et al. Subcutaneous administration of interferon beta-1a for COVID-19: a non-controlled prospective trial. Int Immunopharmacol. 2020;85:106688.
9. Baghaei P, Dastan F, Marjani M, et al. Combination therapy of IFNβ1 with lopinavir–ritonavir, increases oxygenation, survival and discharging of sever COVID-19 infected inpatients. Int Immunopharmacol. 2021;92:107329.
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2021 Pro Pharma Communications International. All Rights Reserved.
News
FDA approves third interchangeable ranibizumab biosimilar Nufymco
FDA approves Poherdy (first interchangeable pertuzumab) and Armlupeg (pegfilgrastim) biosimilars
General
Samsung Bioepis wins Pyzchiva case; Regeneron patent rulings threaten foreign biosimilars
Chinese biosimilars go global: growth, partnerships, and challenges
What is the future for the US biosimilar interchangeability designation

Biosimilars/Research Posted 05/06/2025
Biosimilar clinical efficacy studies: are they still necessary?

Biosimilars/Research Posted 27/05/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment