In Brazil, the registration of new drugs is carried out only when the regulatory agency (Agência Nacional de Vigilância Sanitária, Anvisa) is fully satisfied with their quality, efficacy and safety. Likewise for biosimilars it is necessary that the biosimilar be equally effective and safe and without contaminants in relation to the originator medicine.
Biosimilar low molecular weight heparins in Brazil
Biosimilars/Research
|
Posted 15/04/2011
 0
Post your comment
0
Post your comment

Preclinical studies in vitro and in vivo, verification of raw material origin and phase I, II and III clinical studies are recommended for registration of biosimilars in the international market. A review carried out by Adjunct Professor Yoshida at the Medical School of Universidade Estadual Paulista ‘Júlio de Mesquita Filho’, Botucatu, Brazil, has shown that the tests necessary for the registration of biosimilars must follow strict international protocols because of the variability of biological products, particularly low molecular weight heparins (LMWHs).
Heparin and LMWHs are heterogeneous mixtures of glycosamino-glycans which are used for the treatment of thrombotic and cardiovascular disorders.
Raw materials
Of major concern when considering LMWHs is the origin of the raw material for the production of heparin. It is therefore important that the type of tissue, animal and country of manufacture are clearly identified in any registration documents submitted to the relevant authorities.
Structure and purity
Comparative studies on structural integrity by magnetic resonance imaging, molecular weight assessment using gel filtration, and anticoagulant potential by anti‑Xa, anti-IIa activities, may be used to indicate the similarity and purity between the originator and the biosimilar.
Preclinical studies
In vitro tests on the biosimilar’s activity must be repeated and must be consistent with those of the originator medicine.
Studies on acute and chronic toxicity, along with anticoagulant potential, in at least two animal species should also be part of the preclinical evaluations, comparing different dosages of the biosimilar and originator product.
Clinical studies
Phase I evaluations in normal volunteers for five to seven days should be performed, followed by investigations in patients with renal failure.
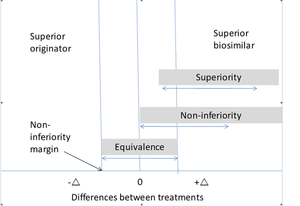
At least one double-blind phase III study which aims to prevent arterial or venous thromboembolism is recommended by the EMA. The phase III studies must assess the biosimilar in relation to the originator product by statistical studies such as superiority, equivalence and non-inferiority trials, see figure.
Non-inferiority trials are the most common type of trial conducted to compare originator medicines and biosimilars, with the non-inferiority margin (Δ) being based on previous studies about the originator product, preferably in comparison to placebo.
The author concluded that scientific evidence and safety must always come before any other interests, other than the patients’ well-being.
Related article
Immunogenicity of biosimilar low molecular weight heparins
Reference
Yoshida BW. Studies on biosimilar medications. J Vasc Bras. 2010;9(3):141-4.
News
FDA approves Poherdy (first interchangeable pertuzumab) and Armlupeg (pegfilgrastim) biosimilars
EMA recommends approval for insulin glargine biosimilar Ondibta and denosumab biosimilar Osqay
General
Samsung Bioepis wins Pyzchiva case; Regeneron patent rulings threaten foreign biosimilars
Chinese biosimilars go global: growth, partnerships, and challenges
What is the future for the US biosimilar interchangeability designation

Biosimilars/Research Posted 05/06/2025
Biosimilar clinical efficacy studies: are they still necessary?

Biosimilars/Research Posted 27/05/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment