A consensus has emerged that approval of biosimilars requires both biological and clinical evidence. The ‘comparability exercise’ requires consideration of a wide range of aspects, including analytical and physico-chemical characterisation by several methods, comparative biological assays, comparative immunogenicity assessment, among others. The use of different host cells for the biosimilar product and the comparator in principle is possible.
Approval of biosimilar epoetins: how similar are they?
Biosimilars/Research
|
Posted 24/09/2010
 0
Post your comment
0
Post your comment

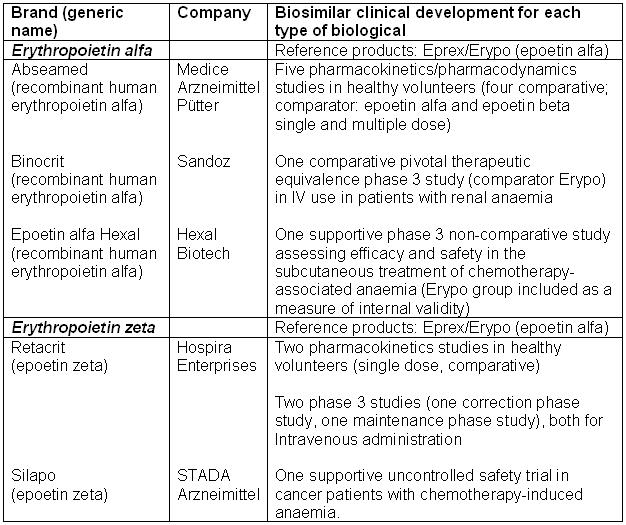
Although required, a clinical programme for a biosimilar should have the primary aim of establishing ‘similarity’ and thus might be abridged compared with full clinical development of a new drug. The extent of studies required depends on the complexity of the product, its mode of action, its toxicity, the possible clinical effect of immunogenicity and the comparability of the response in different populations. See Table 1.
Table 1: Biosimilar erythropoietins approved in the EU in 2007
The high standards manufacturers of follow-up biologics have to meet require extensive investment in product testing, production and marketing. Consequently, the cost of protein drugs will continue to be relatively high.
Related article
Biosimilar epoetins: how similar are they?
Reference
Schellekens H. Biosimilar epoetins: how similar are they? Eur J Hosp Pharm. 2004;10(3):243-7.
News
EMA recommends approval for insulin glargine biosimilar Ondibta and denosumab biosimilar Osqay
FDA approves denosumab biosimilars Osvyrti and Jubereq, Boncresa and Oziltus
General
Samsung Bioepis wins Pyzchiva case; Regeneron patent rulings threaten foreign biosimilars
Chinese biosimilars go global: growth, partnerships, and challenges
What is the future for the US biosimilar interchangeability designation

Biosimilars/Research Posted 05/06/2025
Biosimilar clinical efficacy studies: are they still necessary?

Biosimilars/Research Posted 27/05/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment