Biosimilars/Research
Clinical trials for etanercept biosimilars
Researchers from the Charité – Universitätsmedizin Berlin, Germany, carried out a systematic review into clinical trials for etanercept biosimilars as part of their investigation into biosimilars for the treatment of psoriasis [1].
Clinical trials for infliximab biosimilars
A systematic review into clinical trials for infliximab biosimilars was carried out as part of an investigation into biosimilars for the treatment of psoriasis by researchers from the Charité – Universitätsmedizin Berlin, Germany [1].
Incomplete processing in recombinant streptokinase
A study investigating the impact of incomplete processing on streptokinase activity has found that ‘similar biologics’ or different batches of the biological may have different potencies, depending on the degree of amino-terminal methionine processing and on the pharmacopoeial assay method used, affecting the dosage patients receive [1].
Variation in recombinant streptokinase
A study of blood clot dissolving drug streptokinase has highlighted the mantra of ‘the process is the product’ in the case of biologicals [1].
Clinical trials for adalimumab biosimilars
As part of their investigation into biosimilars for the treatment of psoriasis, researchers from the Charité – Universitätsmedizin Berlin, Germany, carried out a systematic review into clinical trials for adalimumab biosimilars [1].
Biosimilar filgrastim highly similar to originator filgrastim
A study comparing Sandoz’s filgrastim biosimilar (Zarzio) with originator filgrastim (Neupogen) has shown that they are highly similar in terms of their structure and function [1].
Clinical trials of biosimilars for psoriasis treatment
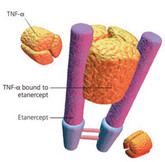
Tumour necrosis factor-alpha (TNF-alpha) is a cytokine, or protein, that prompts the body to create inflammation. In psoriasis and psoriatic arthritis, there is excess production of TNF-alpha in the skin or joints. Therefore, drugs that block TNF-alpha, such as Enbrel (etanercept), Humira (adalimumab), Remicade (infliximab) and Stelara (ustekinumab), can be used to treat psoriasis and psoriatic arthritis.
Considerations when promoting generics prescribing in the US
Sarpatwari and co-authors investigate the legal and ethical consideration for promoting generics prescribing in the US and how this might apply to biosimilars [1].
Adalimumab biosimilar meets primary endpoints in phase I study
US biotech company Oncobiologics announced on 12 February 2015 that ONS-3010, its adalimumab biosimilar candidate, met the primary endpoints in its first clinical study.
Promoting generics prescribing in the US
Sarpatwari and co-authors discuss physician-centered strategies used to promote generics prescribing in the US and how such strategies might apply to biosimilars [1].