Biosimilars/Research
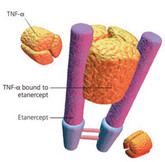
Positive phase III results for etanercept biosimilar
On 9 November 2015, biopharmaceutical specialists Coherus and Baxalta (a spin-off company from Baxter International) announced positive results from a phase III study of its etanercept biosimilar (CHS-0214).
Biosimilar naming conventions around the world
Naming conventions from around the world was one of the topics investigated in a study by researchers from the Tufts Center for the Study of Drug Development (Tufts CSDD) [1].
Positive phase III results for Samsung Bioepis biosimilars
Results of phase III clinical studies of candidate etanercept biosimilar SB4 infliximab biosimilar SB2 and adalimumab biosimilar SB5 have all met their primary endpoints, according to an announcement on 7 November 2015 by Samsung Bioepis (a Biogen and Samsung joint venture).
Biosimilars and interchangeability
In a study by researchers from the Tufts Center for the Study of Drug Development (Tufts CSDD) the question of interchangeability around the world was one of the topics investigated [1].
Economic considerations for rheumatoid arthritis biosimilars
In the paper by Gulácsi et al. [1], the authors stated that biosimilars have the potential to reduce costs and increase patient access to biologicals in the treatment of rheumatoid arthritis (RA) and other chronic inflammatory rheumatic and bowel diseases.
Non-originator low molecular weight heparins: generics or biosimilars
First developed in the 1980s, low molecular weight heparins (LMWHs) are complex anticoagulants derived from porcine intestinal mucosa and are used in the treatment of deep vein thrombosis and pulmonary embolism. Heparin sodium’s anticoagulant activity is mainly governed by its ability to bind to the serine protease inhibitor antithrombin (AT). In this sense, heparin sodium functions as a cofactor for AT by modifying its conformation, thereby accelerating the inactivation of clotting factors.
Positive phase I results for adalimumab biosimilar
In an announcement on 28 October 2015, biopharmaceutical specialist Boehringer Ingelheim Pharmaceuticals (Boehringer Ingelheim) claimed that results from a phase I study of its adalimumab biosimilar (BI 695501) had ‘demonstrated pharmacokinetic bioequivalence’ to Humira (adalimumab).
Biosimilar naming and AE reporting
How will biosimilar naming affect the ability to attribute adverse events (AEs) to a particular product? This is a question researchers from the Tufts Center for the Study of Drug Development (Tufts CSDD) sought to answer [1].
Rituximab biosimilar claimed similar pharmacokinetics to originator
A phase I/II study of Pfizer’s rituximab biosimilar candidate PF-05280586 has shown similar pharmacokinetic (PK) properties, according to Jacobs and co-authors [1].
Biosimilars use in Italy increasing
A study of the use of biosimilars in four large Italian geographic areas has found that the use of biosimilar erythropoiesis-stimulating agents (ESAs), especially in naïve patients, increased significantly during the years 2009–2013 [1].