The development of a biosimilar version of Rituxan (rituximab) is proceeding as planned, Sandoz’s Global Head, Mr Jeff George told Bloomberg.
Sandoz’s biosimilar rituximab on track
Biosimilars/News
|
Posted 29/03/2013
 0
Post your comment
0
Post your comment
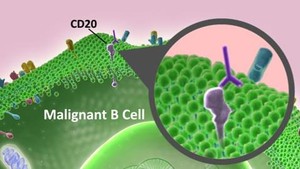
Mr George said ‘our program[me] is on track, but we’ve never given a time line as to when we are coming to market with the product.’ This disputes earlier claims by Roche’s Chief Executive Officer Dr Severin Schwan, who said in January 2013 that the introduction of Sandoz’s product may be delayed until 2016. Although Mr George adds that ‘a number of competitors have faced issues with their clinical trial program[me]s’, possibly referring to Teva Pharmaceutical Industries having to stop their phase III trial and Samsung halting its clinical development, both in October 2012 [1, 2].
Rituximab is a chimeric mouse-human monoclonal antibody that binds to the CD20 antigen presented on the B-cell surface. It is indicated for treatment of lymphomas, autoimmune diseases and transplant rejection.
Sandoz started a phase II clinical trial for the two most important indications of rituximab, rheumatoid arthritis and non-Hodgkin’s lymphoma, in January 2011 [3].
Sandoz’s biosimilar will be a potential competitor to Switzerland-based Roche’s originator brand-name drugs Rituxan and MabThera. Rituximab ranks among the top three biological (biopharmaceutical) drugs worldwide, with 2012 sales of about US$7 billion.
The patent on rituximab does not expire until 2015, despite this fact, other companies, including Boehringer Ingelheim [4], Stada Arzneimittel and Gedeon Richter [5], are also reported to be working on biosimilar versions of the drug. iBio, self-proclaimed leader in the plant-made pharmaceutical field, also announced in October 2011 that it had produced rituximab in non-transgenic green plants, paving the way for lower cost production methods for biosimilars [6].
Related articles
Sandoz starts phase III US trial for biosimilar epoetin alfa
Sandoz starts phase III trials for biosimilar filgrastim and pegfilgrastim
References
1. GaBI Online - Generics and Biosimilars Initiative. Teva halts phase III biosimilar rituximab trial [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2013 Mar 29]. Available from: www.gabionline.net/Biosimilars/News/Teva-halts-phase-III-biosimilar-rituximab-trial
2. GaBI Online - Generics and Biosimilars Initiative. Samsung halts biosimilar rituximab development www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2013 Mar 29]. Available from: www.gabionline.net/Biosimilars/News/Samsung-halts-biosimilar-rituximab-development
3. GaBI Online - Generics and Biosimilars Initiative. Sandoz announces biosimilar rituximab [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2013 Mar 29]. Available from: www.gabionline.net/Biosimilars/News/Sandoz-announces-biosimilar-rituximab
4. GaBI Online - Generics and Biosimilars Initiative. Boehringer Ingelheim starts biosimilar rituximab trial [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2013 Mar 29]. Available from: www.gabionline.net/Biosimilars/Research/Boehringer-Ingelheim-starts-biosimilar-rituximab-trial
5. GaBI Online - Generics and Biosimilars Initiative. Stada and Richter to collaborate on biosimilar development [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2013 Mar 29]. Available from: www.gabionline.net/Biosimilars/News/Stada-and-Richter-to-collaborate-on-biosimilar-development
6. GaBI Online - Generics and Biosimilars Initiative. Rituximab biosimilar successfully produced in plants [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2013 Mar 29]. Available from: www.gabionline.net/Biosimilars/News/Rituximab-biosimilar-successfully-produced-in-plants
Permission granted to reproduce for personal and educational use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Source: Bloomberg
Research
Reaching ESG goals in pharmaceutical development
What is the future for the US biosimilar interchangeability designation
General
Samsung Bioepis wins Pyzchiva case; Regeneron patent rulings threaten foreign biosimilars
Chinese biosimilars go global: growth, partnerships, and challenges
Most viewed articles
The best selling biotechnology drugs of 2008: the next biosimilars targets
Global biosimilars guideline development – EGA’s perspective
Related content
FDA approves Poherdy (first interchangeable pertuzumab) and Armlupeg (pegfilgrastim) biosimilars
EMA recommends approval for insulin glargine biosimilar Ondibta and denosumab biosimilar Osqay
FDA approves denosumab biosimilars Osvyrti and Jubereq, Boncresa and Oziltus
FDA approves aflibercept biosimilar Eydenzelt and label expansion for adalimumab biosimilar Yuflyma
FDA approves Poherdy (first interchangeable pertuzumab) and Armlupeg (pegfilgrastim) biosimilars

Biosimilars/News Posted 27/01/2026
EMA recommends approval for insulin glargine biosimilar Ondibta and denosumab biosimilar Osqay

Biosimilars/News Posted 16/01/2026
FDA approves denosumab biosimilars Osvyrti and Jubereq, Boncresa and Oziltus

Biosimilars/News Posted 07/01/2026
FDA approves aflibercept biosimilar Eydenzelt and label expansion for adalimumab biosimilar Yuflyma

Biosimilars/News Posted 05/12/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets






Post your comment