Partners Eli Lilly and Boehringer Ingelheim, announced on 10 September 2014 that they had received European Commission (EC) approval for its biosimilar insulin glargine product Abasria (LY2963016), a first for Europe.
European approval for biosimilar insulin
Biosimilars/News
|
Posted 12/09/2014
 0
Post your comment
0
Post your comment
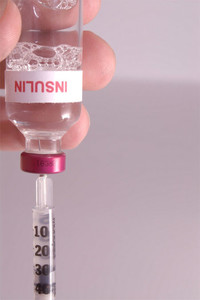
The news follows the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) positive recommendation of 27 June 2014 [1].
The biosimilar insulin glargine product is a basal (long-acting) insulin with the same amino acid sequence as French drugmaker Sanofi’s diabetes drug Lantus (insulin glargine). Abasria is intended to provide long-lasting blood sugar control between meals and at night and will be available in a pre-filled pen and cartridges for a reusable pen.
The marketing authorization for Abasria is based upon a comprehensive clinical data programme, which showed it has similar efficacy and safety compared to Lantus in people with type 1 and type 2 diabetes. The application included results from pharmacokinetic and pharmacodynamic studies, as well as phase III studies in patients with type 1 and type 2 diabetes.
This is the first biosimilar insulin to receive marketing authorization in Europe, but it will not be the last. Other companies working on biosimilar insulin products include generics giant Mylan and India-based Biocon [2], as well as Sanofi [3].
Eli Lilly and Boehringer Ingelheim also received tentative approval for their insulin glargine product (LY2963016) from the US Food and Drug Administration (FDA). The application for approval of the drug, however, which will be called Basaglar in the US, was submitted to FDA using a new drug application and not via the abbreviated biosimilars pathway [4].
Related Articles
EMA reviewing biosimilar insulin application
Marvel withdraws biosimilar insulin applications
References
1. GaBI Online - Generics and Biosimilars Initiative. EMA approves biosimilar insulin [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2014 Sep 12]. Available from: www.gabionline.net/Biosimilars/News/EMA-approves-biosimilar-insulin
2. GaBI Online - Generics and Biosimilars Initiative. Mylan and Biocon to partner on insulin products [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2014 Sep 12]. Available from: www.gabionline.net/Biosimilars/News/Mylan-and-Biocon-to-partner-on-insulin-products
3. GaBI Online - Generics and Biosimilars Initiative. Sanofi starts biosimilar insulin trials [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2014 Sep 12]. Available from: www.gabionline.net/Biosimilars/News/Sanofi-starts-biosimilar-insulin-trials
4. GaBI Online - Generics and Biosimilars Initiative. FDA grants tentative approval for insulin treatment [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2014 Sep 12]. Available from: www.gabionline.net/Biosimilars/News/FDA-grants-tentative-approval-for-insulin-treatment
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2014 Pro PharmaCommunications International. All Rights Reserved.
Source: Eli Lilly, Boehringer Ingelheim
Research
Reaching ESG goals in pharmaceutical development
What is the future for the US biosimilar interchangeability designation
General
Samsung Bioepis wins Pyzchiva case; Regeneron patent rulings threaten foreign biosimilars
Chinese biosimilars go global: growth, partnerships, and challenges
Most viewed articles
The best selling biotechnology drugs of 2008: the next biosimilars targets
Global biosimilars guideline development – EGA’s perspective
Related content
FDA approves third interchangeable ranibizumab biosimilar Nufymco
FDA approves Poherdy (first interchangeable pertuzumab) and Armlupeg (pegfilgrastim) biosimilars
EMA recommends approval for insulin glargine biosimilar Ondibta and denosumab biosimilar Osqay
FDA approves denosumab biosimilars Osvyrti and Jubereq, Boncresa and Oziltus
FDA approves third interchangeable ranibizumab biosimilar Nufymco

Biosimilars/News Posted 09/02/2026
FDA approves Poherdy (first interchangeable pertuzumab) and Armlupeg (pegfilgrastim) biosimilars

Biosimilars/News Posted 27/01/2026
EMA recommends approval for insulin glargine biosimilar Ondibta and denosumab biosimilar Osqay

Biosimilars/News Posted 16/01/2026
FDA approves denosumab biosimilars Osvyrti and Jubereq, Boncresa and Oziltus

Biosimilars/News Posted 07/01/2026
The best selling biotechnology drugs of 2008: the next biosimilars targets






Post your comment