Biosimilars/News
Korean approval for infliximab biosimilar
Samsung Bioepis and partner Merck announced on 4 December 2015 that Samsung Bioepis had received approval for its infliximab biosimilar Renflexis from the Korean Ministry of Food and Drug Safety (MFDS, formerly the Korea Food and Drug Administration).
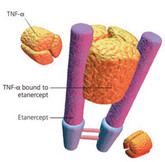
EMA recommends approval of etanercept biosimilar
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) announced on 19 November 2015 that it had recommended granting of marketing authorization for a biosimilar etanercept product (SB4).
Biosimilar etanercept approved in South Korea
US pharma giant Merck and South Korean biosimilars maker Samsung Bioepis announced on 8 September 2015 the approval of their biosimilar etanercept product Brenzys by the Ministry of Food and Drug Safety (MFDS) in Korea.
Samsung adds 48-week extension to SB4 biosimilar study
Samsung Bioepis has added a 48-week extension to the phase III study of its candidate etanercept biosimilar SB4 in rheumatoid arthritis patients.
Merck outlines biosimilars programme
During the 35th Annual Health Care Conference held on 2−4 March 2015 in Boston, USA, US pharma giant Merck outlined its biosimilars programme.
Samsung Bioepis submits second biosimilar to EMA
South Korean electronics giant Samsung and biotechnology company Biogen Idec’s joint venture Samsung Bioepis announced on 13 March 2015 that its infliximab biosimilar candidate, SB2, had been accepted for review by the European Medicines Agency (EMA).
Biosimilar etanercept submitted for approval in EU
South Korean electronics giant Samsung and biotechnology company Biogen Idec’s joint venture Samsung Bioepis announced on 21 January 2015 that its etanercept biosimilar candidate, SB4, had been accepted for review by the European Medicines Agency (EMA).
Biogen and Samsung make deal for marketing anti-TNF biosimilars
US biotechnology company Biogen Idec (Biogen) and Korean electronics giant Samsung announced on 17 December 2013 that Biogen has exercised its right to enter into an agreement to commercialize anti-tumour necrosis factor (TNF) biosimilar product candidates in Europe.
Trials to start for biosimilar infliximab and etanercept
Pfizer is to start a phase I study for its biosimilar infliximab candidate (PF-06438179) and Samsung Bioepis has initiated a phase III clinical trial for its biosimilar etanercept (SB4), according to entries on the US and EU clinical trials websites.
BioOutsource launches ready-to-use biosimilarity assays
Sartorius Stedim BioOutsource (BioOutsource), a subsidiary of Sartorius Stedim Biotech, has launched a range of ready-to-use assays for testing biosimilarity. The assays are available for biosimilars of Hoffmann–La Roche/Chugai’s rheumatoid arthritis treatment Actemra (tocilizumab), Centocor’s psoriasis treatment Stelara (ustekinumab) and Novartis/Genentech’s age-related macular degeneration drug Lucentis (ranibizumab).