Biosimilars/News
Biocon/Mylan launch pegfilgrastim biosimilar Fulphila in Australia
US-based drugmaker Mylan and partner India-based biologicals specialist Biocon have announced the launch of their pegfilgrastim biosimilar, Fulphila, in Australia. The drug can be used to treat neutropenia (a lack of white blood cells) in cancer patients.
Mabion withdraws application for rituximab biosimilar in EU
Polish biologicals company Mabion Spolka Akcyjna (Mabion) has withdrawn its duplicate applications for its rituximab biosimilar, MabionCD20.
Amgevita approved in Colombia
Colombia’s National Institute of Food and Drug Monitoring (INVIMA – Instituto Nacional de Vigilancia de Medicamentos y Alimentos) has approved the first adalimumab producto bioterapéutico similar (similar biotherapeutic product).
Merck launches trastuzumab biosimilar in the US
US pharma giant Merck (known as MSD outside the US and Canada) and Korea-based Samsung Bioepis (Samsung and Biogen’s joint venture) announced in on 15 April 2020 that they had launched their trastuzumab biosimilar, Ontruzant, in the US.
EC approval for rituximab biosimilar Ruxience
On 2 April 2020, pharma giant Pfizer announced that its rituximab biosimilar, Ruxience (PF‑05280586), had received European Commission (EC) approval.
Non-originator biologicals approved in Bosnia and Herzegovina
Russian biotechnology company Biocad announced on 30 March 2020 that it had received approval for two of its anticancer non-originator biologicals in Bosnia and Herzegovina.
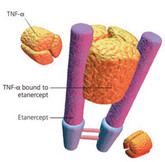
EMA recommends approval of etanercept biosimilar Nepexto
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) announced on 27 March 2020 that it had recommended granting of marketing authorization for a biosimilar etanercept product.
Celltrion/Teva launch trastuzumab biosimilar Herzuma in US
Celltrion and Teva announced in March 2020 that they have launched their trastuzumab biosimilar Herzuma (trastuzumab-pkrb) in the US. Herzuma can be used to treat breast and gastric cancer and has recently been approved in Canada [1].
Celltrion launches infliximab biosimilar Remsima SC in Europe
In a successful time for Celltrion Healthcare (Celltrion), the company has launched their infliximab biosimilar Remsima in Germany and the UK.
Amgen starts phase III trial for aflibercept biosimilar
US-based biotech giant Amgen is initiating a phase III clinical trial for a biosimilar of Regeneron’s Eylea (aflibercept).