Biosimilars/News
Mabpharm gains approval for infliximab biobetter in China
US-based biopharmaceutical company Sorrento Therapeutics (Sorrento) announced on 20 July 2021 that its partner, China-based Mabpharm, had received marketing approval from China’s National Medical Products Administration (NMPA), formerly the China Food and Drug Administration (CFDA), for its infliximab ‘biobetter’ (CMAB008).
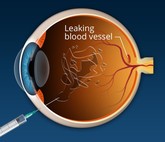
Formycon/Bioeq submit European marketing authorization for ranibizumab biosimilar
German-headquartered companies Formycon and Bioeq have announced a European marketing authorization application for their ranibizumab biosimilar, FYB201.
Human insulin ‘similar biologic’ InsuTrend launched in India
India-based company Anthem Biosciences (AnthemBio) announced that it has launched its human insulin ‘similar biologic’ InsuTrend in India.
EMA recommends approval of Samsung Bioepis/Biogen’s ranibizumab biosimilar Byooviz
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has given a positive opinion for the ranibizumab biosimilar Byooviz (SB11), produced by Samsung Bioepis and commercialized by Biogen.
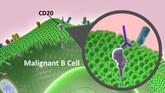
Canada approves rituximab biosimilar Riabni
Canada’s drug regulator, Health Canada, has approved the rituximab biosimilar Riabni for the treatment of rheumatoid arthritis as well as chronic lymphocytic leukaemia and non-Hodgkin’s lymphoma.
Canada approves pegfilgrastim biosimilar Nyvepria
Canada’s drug regulator, Health Canada, has approved the filgrastim biosimilar Nyvepria (PF-06881894). The drug can be used to treat neutropenia (a lack of white blood cells) in cancer patients.
FDA accepts application for Lupin’s pegfilgrastim biosimilar
India-based generics manufacturer Lupin Pharmaceuticals (Lupin) announced on 2 June 2021 that the US Food and Drug Administration (FDA) had accepted the application for approval for its proposed pegfilgrastim biosimilar.
Trastuzumab emtansine ‘similar biologic’ Ujvira launched in India
India-based generics manufacturer Zydus Cadila (Zydus) announced on 24 May 2021 the launch of its trastuzumab emtansine ‘similar biologic’ Ujvira in India. The launch, according to Zydus ‘marks the world’s first biosimilar antibody drug conjugate (ADC) of trastuzumab emtansine’.
Clinical trials for aflibercept biosimilars
Sandoz, the generics division of Novartis, has announced the start of a phase III clinical trial of its aflibercept (Eylea) biosimilar, a treatment for age-related macular degeneration. Clinical trials for a number of competitive biosimilars are also underway.
Argentina approves bevacizumab similar biological medicine Zutrab
The Argentina-based Richmond Laboratory (Laboratorios Richmond) announced on 19 March 2021 that it had gained approval from Argentina’s National Administration of Drugs, Foods and Medical Devices (Administración Nacional de Medicamentos, Alimentos y Tecnología Médica, ANMAT) for its similar biological medicine (medicamento biológico similar) Zutrab (bevacizumab).