Biosimilars/News
Applications for natalizumab biosimilars accepted by FDA and EMA
Applications for natalizumab biosimilars made by Sandoz (the generics division of Novartis) and Polpharma Biologics (Polpharma) have been accepted by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA).
FDA accepts application for tocilizumab biosimilar
Fresenius Kabi, the generics unit of German healthcare giant Fresenius, announced on 1 August 2022 that the US Food and Drug Administration (FDA) had accepted the application for their proposed tocilizumab biosimilar (MSB11456).
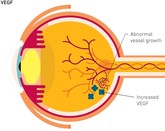
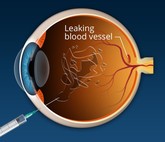
FDA approves first interchangeable ranibizumab biosimilar
Polpharma Biologics, Formycon and Bioeq jointly announced on 2 August 2022 that they had received approval from the US Food and Drug Administration (FDA) for their ranibizumab biosimilar (CHS-201/FYB201).
Innovent-Etana bevacizumab biosimilar approved in Indonesia
Innovent announced the approval of its bevacizumab biosimilar in Indonesia, that was developed in partnership with PT Etana Biotechnologies Indonesia.
Ranibizumab biosimilar, FYB201, receives EMA recommendation
Formycon’s ranibizumab biosimilar, FYB201, has received a positive opinion from the European Medicines Agency (EMA) Committee for Medicinal Products for Human Use (CHMP).
New data on infliximab and adalimumab biosimilars at EULAR 2022
The Annual European Congress of Rheumatology (EULAR 2022) was held at the beginning of June 2022. This platform was used to announce the results of a study that showed that, in patients with rheumatoid arthritis, statistically greater improvements in clinical outcomes with subcutaneous infliximab, compared to intravenous infliximab. In addition, Samsung Bioepis announced new data on their established adalimumab biosimilar Imraldi (Hadlima outside Europe).
EMA accepts application for high concentration adalimumab biosimilar
Sandoz, the generics division of Novartis, announced on 17 June 2022 that the European Medicines Agency (EMA) has accepted the application for its high concentration formulation (HCF) of its adalimumab biosimilar Hyrimoz (GP2017).
EMA recommends approval of bevacizumab biosimilar Vegzelma
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) announced on 24 June 2022 that it had recommended granting marketing authorization for the bevacizumab biosimilar Vegzelma.
FDA approves pegfilgrastim and rituximab biosimilars
The US Food and Drug Administration (FDA) has approved the pegfilgrastim biosimilar Fylnetra (pegfilgrastim-pbbk) and the rituximab biosimilar Riabni (rituximab-arrx).
Byooviz: first ophthalmology biosimilar launches in US
Biogen and Samsung Bioepis have launched Byooviz (ranibizumab-nuna) on the US market as the first ophthalmology biosimilar. It references Roche’s (Genentech’s) blockbuster therapy, Lucentis.