Biosimilars
EC biosimilar approvals: Omlyclo, Jubbonti/Wyost, and Pyzchiva
The European Commission (EC) granted marketing authorization for four biosimilars. They are Celltrion’s Omlyclo (omalizumab), Sandoz’s denosumab biosimilars Jubbonti and Wyost, and Samsung Bioepis’ Pyzchiva (ustekinumab). Furthermore, the EC approves expanded indication for Samsung Bioepis' Epysqli (eculizumab) to include treatment of atypical haemolytic uremic syndrome (aHUS).
Tocilizumab and pembrolizumab biosimilar advances for Korean firms
In June 2024, Celltrion announced promising results from their phase III study for CT-P47, a biosimilar candidate of RoActemra (tocilizumab), in patients with rheumatoid arthritis (RA). Additionally, the company has submitted an investigational new drug (IND) application to the US Food and Drug Administration (FDA) for the phase III clinical trial of CT-P51, its Keytruda (pembrolizumab) biosimilar. This announcement follows the news that in April 2024, rival Korean biologicals company Samsung Bioepis initiated a phase III clinical trial for SB27, their pembrolizumab biosimilar.
Boehringer Ingelheim to expand access to adalimumab biosimilar
On 13 May 2024, Boehringer Ingelheim (Boehringer) announced an agreement with Quallent Pharmaceuticals, a private label pharmaceutical distributor, to help expand patient access to citrate-free adalimumab-adbm, Boehringer’s biosimilar to AbbVie’s Humira (adalimumab) in the US.
EMA recommends approval of biosimilar bevacizumab Avzivi
On 30 May 2024, the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion recommending the granting of marketing authorization for Avzivi (bevacizumab), Bio-Thera Solutions’ (Bio-Thera) biosimilar of the reference product Avastin.
Challenges and progress in the registration of biosimilars in Latin America
Biosimilar medicines offer an effective and economical alternative to biotechnological medicines, with a rapidly expanding global market. However, Latin America still faces several significant challenges. The obstacles and advancements experienced in recent years are detailed below.
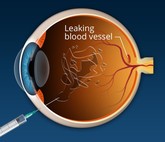
FDA approves first interchangeable aflibercept biosimilars to treat macular degeneration
On 20 May 2024, the US Food and Drug Administration (FDA) approved Biocon Biologic’s Yesafili (aflibercept-jbvf) and Samsung Bioepis and Biogen's Opuviz (aflibercept-yszy) as the first interchangeable biosimilars to Regeneron and Bayer's Eylea (aflibercept). The Samsung Bioepis product has also been launched in South Korea under brand name, Afilivu.
Canada's path to biosimilar adoption and healthcare accessibility: insights from British Columbia
Canada’s British Columbia transitioned to biosimilars in November 2019, achieving significant cost savings reinvested in BC PharmaCare. This initiative has improved drug coverage, expanding access to various medications and medical devices.
FDA approves ustekinumab, trastuzumab, and tocilizumab biosimilars
In April 2024, the US Food and Drugs Administration (FDA) approved Alvotech and Teva’s Selarsdi (ustekinumab-aekn), a biosimilar to Stelara and Shanghai Henlius Biotech’s Herceptin biosimilar, Hercessi (trastuzumab-strf). This comes after the March 2024 news of the approval of Fresenius Kabi’s Tyenne (tocilizumab-aazg), the first tocilizumab biosimilar with an intravenous and subcutaneous formulation.
Update of biosimilars approved by Health Canada in 2023
Since November 2023, Canada’s drug regulator, Health Canada, has approved no fewer than five biosimilars for the treatment of deep vein thrombosis (DVT), cancer, plaque psoriasis, psoriatic arthritis, Crohn’s disease, ulcerative colitis and several serious retinal diseases, such as wet age-related macular degeneration.
EMA recommends approval of biosimilar tocilizumab Tofidence and ustekinumab Wezenla
On 25 April 2024, the European Medicine Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) adopted positive opinions for two biosimilar medicines: Tofidence (tocilizumab) and Wezenla (ustekinumab), recommending the granting of marketing authorization. This comes following news that Wezlana reached the market in Canada in early March 2024.