Biosimilars
Fight continues over biosimilar naming standards
Biosimilar products marketed in the US should share the same common non-proprietary name as the reference brand-name biological medicine, according to the Generic Pharmaceutical Association (GPhA).
The future of biosimilar mAbs in Europe
Biosimilars – products that are similar to originator biological medicinal products – have had a positive impact on healthcare systems. But it takes up to four years following market approval before biosimilars are accepted by the clinical community and by the people holding the purse strings. Now, a new class of biosimilar –monoclonal antibodies (mAbs) – is set to challenge the system further, writes Professor Andrea Laslop of the Austrian Agency for Health and Food Safety [1].
Amgen to start phase III trial for biosimilar adalimumab
Biotechnology giant Amgen is to start a phase III clinical trial for a biosimilar version of adalimumab in patients suffering from severe rheumatoid arthritis according to the EU Clinical Trials Register.
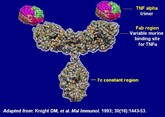
Challenges and opportunities for anticancer mAbs
Several best-selling monocloncal antibodies (mAbs) are due to lose patent protection; presenting regulatory authorities with a complex set of challenges as they prepare for the arrival of novel biosimilars, note Ebbers and co-authors at Utrecht University, The Netherlands [1].
EMA approves biosimilar follitropin alfa and somatropin
The European Medicines Agency’s (EMA’s) Committee for Medicinal Products for Human Use (CHMP) announced on 31 July 2013 that it had recommended granting of marketing authorization for a biosimilar follitropin alfa. While on 9 September 2013, the agency announced the approval of a new somatropin biosimilar.
Failed biosimilars company to be sold
The assets of biosimilars developer Elona Biotechnologies (Elona) will be auctioned on 27 September 2013 unless sold before 6 September 2013.
EC approves first monoclonal antibody biosimilar
Hospira announced on 10 September 2013 that it had received European Commission (EC) approval for its biosimilar monoclonal antibody infliximab (Inflectra), a first in Europe.
Celltrion applies for biosimilar infliximab approval in Japan
On 11 September 2013, South Korean biotechnology company Celltrion announced that it had filed for approval of Remsima, its biosimilar infliximab monoclonal antibody, with Japan’s Ministry of Health, Labour and Welfare (MHLW).
Positive phase III data for Epirus infliximab biosimilar
US-based Epirus Biopharmaceuticals (Epirus) announced on 28 August 2013 that its biosimilar infliximab candidate had demonstrated ‘clinical comparability’ to Remicade as measured by the ACR20 response in severe rheumatoid arthritis patients.
Biocad signs deal for biosimilar darbepoetin alpha in Turkey
Russian biosimilars manufacturer Biocad has signed a definitive agreement with Turkish oncology specialist Koçak Farma for the export of biosimilar darbepoetin alpha active pharmaceutical ingredients (APIs).