Biosimilars
Biosimilars of infliximab
Last update: 27 November 2020
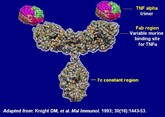
Infliximab is a chimeric monoclonal antibody against tumour necrosis factor alpha (TNF-α). It is used to treat autoimmune diseases, such as ankylosing spondylitis, Crohn’s disease, psoriasis, psoriatic arthritis, rheumatoid arthritis, and ulcerative colitis.
Torrent licenses three ‘similar biologics’ from Reliance
Torrent Pharmaceuticals (Torrent) announced on 25 December 2014 that it had made an exclusive licensing agreement with fellow Indian drugmaker Reliance Life Sciences (Reliance) for the marketing of three ‘similar biologics’ in India.
Impact of nephrology subsequent entry biologics in Canada
Subsequent entry biologics (SEBs) may soon be a reality in Canadian nephrology practice. Along with opportunities to reduce healthcare costs, these agents pose unique challenges to successful implementation. Understanding the experiences around the globe in both regulatory affairs and implementation will be a valuable guide for Canadian clinicians. This review should assist clinicians and policymakers to navigate this complex subject and to make informed decisions in the best interest of their patients [1].
Hanwha to transfer biosimilar etanercept technology to Merck KGaA
South Korea’s Hanwha Chemical Corporation (Hanwha) has reportedly signed a contract with German drug maker Merck KGaA (Merck Group) to export the technology to make its biosimilar etanercept drug.
Japanese approval for insulin glargine biosimilar
Partners Eli Lilly and Boehringer Ingelheim confirmed on 19 January 2015 that they had received Japanese regulatory approval for their biosimilar insulin glargine product (LY2963016).
No relevant difference in ADRs from biosimilars and originators
A study of adverse drug reactions reported in Italy has shown no difference between the number and type of side effects reported for biosimilars and their corresponding originators [1].
Biosimilars applications under review by EMA – December 2014
The European Medicines Agency (EMA) is the body responsible for approval of biosimilars within the European Union (EU). A legal framework for approving biosimilars was established in 2003. Approval of biosimilars is based on an abbreviated registration process, which allows biosimilars manufacturers to provide a reduced package of information compared to originator drugs, provided they can prove ‘similarity’ to the originator or reference drug.
Adalimumab similar biologic launched in India
Indian generics maker Zydus Cadila announced on 9 December 2014 the launch of its adalimumab similar biologic in India.
Biosimilar etanercept submitted for approval in EU
South Korean electronics giant Samsung and biotechnology company Biogen Idec’s joint venture Samsung Bioepis announced on 21 January 2015 that its etanercept biosimilar candidate, SB4, had been accepted for review by the European Medicines Agency (EMA).
Filgrastim biosimilar has similar safety and efficacy to Neupogen
A filgrastim biosimilar (EP2006) from Sandoz, the generics division of Swiss pharma giant Novartis, has shown similar safety and efficacy compared to Amgen’s Neupogen (filgrastim) in a pivotal phase III clinical study.