Formycon’s ranibizumab biosimilar, FYB201, has received a positive opinion from the European Medicines Agency (EMA) Committee for Medicinal Products for Human Use (CHMP).
On 24 June 2022, German biotech company Formycon received a positive opinion from the CHMP for their ranibizumab biosimilar, FYB201.
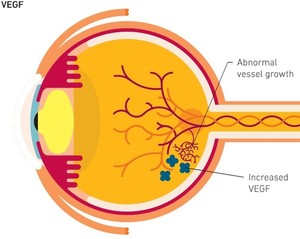
Ranibizumab is a monoclonal antibody fragment created from the same parent mouse antibody as bevacizumab. Ranibizumab inhibits angiogenesis (the formation of new blood vessels) by inhibiting vascular endothelial growth factor A (VEGF-A), a mechanism similar to bevacizumab [1]. It can be used for the treatment of wet age-related macular degeneration (AMD), and is sold under the brand name Lucentis by originator company Genentech [2].
The EMA CHMP recommended the biosimilar FYB201 for the treatment of wet AMD as well as several other ocular diseases including diabetic macular oedema (DME), proliferative diabetic retinopathy (PDR), macular oedema due to retinal vein occlusion (branch RVO or central RVO) and choroidal neovascularization (CNV).
The recommendation is based on data including a randomized, double-blind, multicentre, parallel-group phase III study. In this study, FYB201 demonstrated comparable efficacy, safety, pharmacokinetics and immunogenicity to Lucentis in patients with wet AMD.
This decision will form the basis for the European Commission’s decision to grant marketing authorization for FYB201, which is expected at the end of August 2022.
FYB201 was approved by the UK Medicines and Healthcare products Regulatory Agency (MHRA) earlier this year [3], with commercialization partner Teva to launch the product under the trade name Ongavia – the first Lucentis/ophthalmic biosimilar to be available in the UK.
This follows an update on Formycon’s other projects, published by the company on 17 June 2022. This update confirmed that FYB201 is likely to be the first Lucentis/ophthalmic biosimilar to reach European markets.
Formycon is also developing a ustekinumab biosimilar, currently named FYB202, with treatment of the last patient in a phase III clinical trial being successfully completed and results expected in July 2022.
Formycon’s aflibercept biosimilar candidate has also enrolled its last patient in an ongoing phase III trial, which aims to demonstrate similarity to the reference product Eylea in patients with AMD.
Finally, the company is also developing a SARS-CoV-2 antiviral named FYB207, which is a fusion protein of the human protein ACE2, the entry point for cell infection. FYB207 has been shown to neutralize the omicron variant of SARS-CoV-2 in vitro and recent developments have increased its in vivo half-life and efficacy. An accelerated development programme is planned, with a clinical trial in preparation.
Related articles
Canada approves ranibizumab biosimilar Byooviz
Phase III trial evidence used in approval of ranibizumab biosimilar Byooviz
Teva signs deal with Bioeq for ranibizumab biosimilar FYB201
References
1. GaBI Online - Generics and Biosimilars Initiative. Biosimilars of bevacizumab [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2022 Jul 22]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-of-bevacizumab
2. GaBI Online - Generics and Biosimilars Initiative. Biosimilars of ranibizumab [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2022 Jul 22]. Available from: www.gabionline.net/biosimilars/general/Biosimilars-of-ranibizumab
3. GaBI Online - Generics and Biosimilars Initiative. Lucentis biosimilars approved in the UK and Korea [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2022 Jul 22]. Available from: www.gabionline.net/biosimilars/news/lucentis-biosimilars-approved-in-the-uk-and-korea
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2022 Pro Pharma Communications International. All Rights Reserved.








 0
0










Post your comment