The first biosimilar infliximab was sold in Canada in 2015, yet by 2018 uptake was still less then 10%, data from the Patented Medicine Prices Review Board (PMPRB) reveal.
Infliximab is a monoclonal antibody treatment for auto-immune diseases including inflammatory bowel disease, rheumatoid arthritis and psoriasis. It is administered by slow injection into a vein and works by binding the inflammatory cytokine tumour necrosis factor-alpha (TNF-α).
Originally sold under the brand name Remicade, biosimilar versions were approved in the European Union in 2013 and in the US in 2016. Infliximab was also one of the first biosimilar medicines to be approved in Canada.
Pfizer’s biosimilar Inflectra was first sold in Canada in 2015. However, according to data from the Canadian PMPRB, uptake has been limited. They report data showing that by the end of 2018 the originator drug Remicade retained significant market share, with Inflectra taking just 8% of the market.
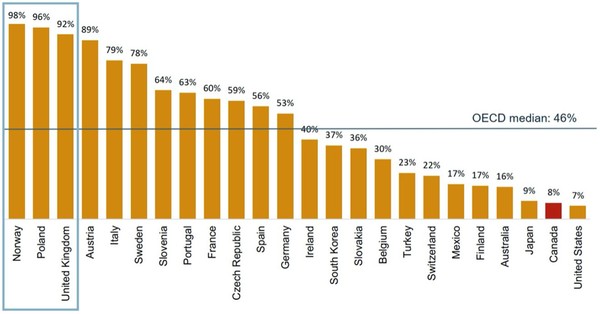
When compared to other countries in the Organisation for Economic Co-operation and Development (OECD), Canada’s biosimilar infliximab uptake is far below the median of 46%, see Figure 1.
By contrast, Norway, Poland and the UK each had over 90% biosimilar infliximab by in the fourth quarter of 2018.
Figure 1: Uptake of infliximab biosimilars (share of units) in OECD countries, Q4-2018
The success of biosimilar uptake in these nations is due to a range of high impact policies. In Norway, for example, physicians are required to prescribe the cheapest medicine and switching to biosimilars is common practice.
Poland also allows biosimilar substitution and the Ministry of Health takes the view that ‘any exchange within the scope of drugs containing infliximab at any level of therapy is permissible’. In the UK, guidance states that nine out of 10 new patients should be started on the best value medicine within three months of a biosimilar launch, and at least 80% of existing patients should be switched to the best value biological within a year.
Further data from Canada shows that only a small number of patients taking Remicade switched to a biosimilar version. Inflectra represented just 1% of patients in 2017/18, despite infliximab being used by 30% of patients taking biological disease-modifying antirheumatic drugs.
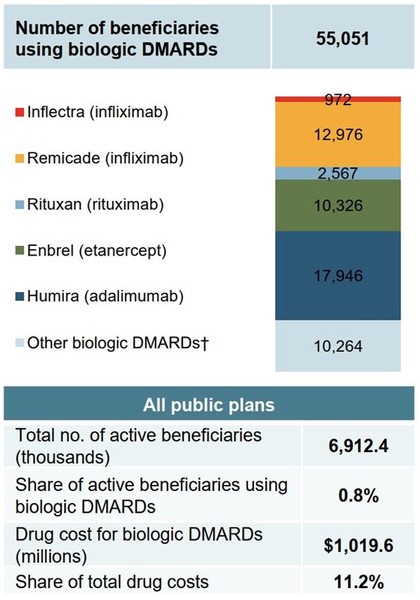
Although patients using biological disease-modifying antirheumatic drugs accounted for less than 1% of eligible beneficiaries in public plans in 2017/18, they accounted for 11.2% of total drug costs, see Figure 2.
Figure 2: Distribution of patients on biological disease-modifying antirheumatic drugs in Canada, public drug plans, 2017/18 (in CA$)
Source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
This demonstrates the cost savings that could be made by switching such patients to biosimilar versions of their medications. Initiatives to encourage this are already underway in Canada, where three provinces have introduced policies to switch patients to biosimilars [1]. Recent data from British Columbia shows that already 28% of patients with inflammatory bowel disease have switched to a biosimilar version of their medication [2].
Related articles
Low levels of biosimilar uptake in Canada
The cost of biologicals in Canada
Analysis of biological sales for OECD countries
Trends in biological drugs in Canada
Use of biosimilar infliximab gives savings for patients
References
1. GaBI Online - Generics and Biosimilars Initiative. Ontario becomes third Canadian province to switch patients to biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Jul 31]. Available from: www.gabionline.net/Biosimilars/General/Ontario-becomes-third-Canadian-province-to-switch-patients-to-biosimilars
2. GaBI Online - Generics and Biosimilars Initiative. British Columbia releases latest data on biosimilar switches [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Jul 31]. Available from: www.gabionline.net/Biosimilars/General/British-Columbia-releases-latest-data-on-biosimilar-switches
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2020 Pro Pharma Communications International. All Rights Reserved.
Source: Government of Canada








 0
0











Post your comment