What factors do patients take into account when deciding to accept or reject generics substitution? This is the question asked in a recent research article [1].
Mandatory generics substitution was introduced in Finland in April 2003. However, individual patients or physicians have the option of refusing the substitution.
Previous studies have suggested that generics substitution may confuse or make people suspicious when, for example, the names, colours, packaging, etc.; of their medication is changed [2, 3]. In fact, FDA has even stated, ‘we really want the generic product to look the same as the innovator as much as possible’, suggesting that any differences are important [4].
This survey of Finnish patients was carried out via postal questionnaire in five counties in Finland during the autumn of 2008 to find out what factors influenced patients’ choice of medicines.
From the 3,000 questionnaires posted 1,844 questionnaires were returned, giving a response rate of 62%, with 55.2% of responders being female. Of the responders, 159 (8.6%) had refused generics substitution, 633 (34.3%) had accepted, 301 (16.3%) had both accepted and refused, and 751 (40.7%) had no experience with generics substitution.
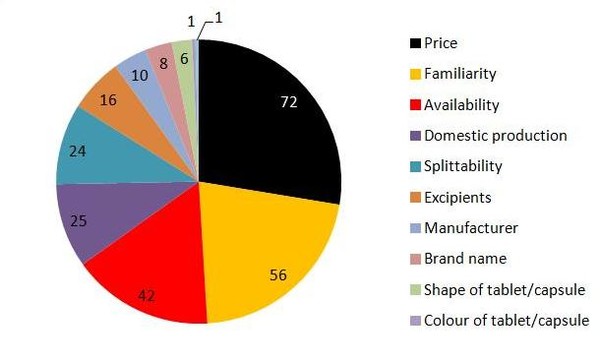
Price (72%), familiarity (56%), and availability (42%) were the three most important factors that influenced patients’ choice of medicines, see Figure 1. Other characteristics of the medicines such as domestic production (25%), splittability (24%), excipients (16%), manufacturer (10%), brand name (8%), shape of the tablet/capsule (6%), colour of the tablet/capsule (1%), and appearance of the package (1%), were not as important to the respondents.
Figure 1: Factors important when patients choose to accept or reject generics substitution
Source: Heikkilä R et al [1]
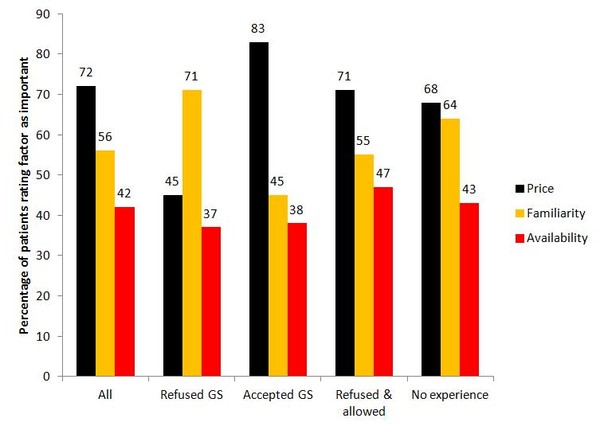
For the people who had refused generics substitution, the familiarity of the medicine was the most important factor (71%). For the people who had accepted generics substitution and for those who had both refused and allowed generics substitution, price was the most important factor (83% and 71%, respectively), see Figure 2.
Figure 2: Factors important when patients choose to accept or reject generics substitution–split by group
Source: Heikkilä R et al [1]
The people who had refused generics substitution tended to be older than those in the other groups. This could be why this group appreciated the familiarity of medicines over price considerations.
Before generics substitution was introduced in Finland it was thought that it would confuse patients. However, this research shows that price, familiarity, and availability were more important for patients in their choice for prescription medicines than the external characteristics of the medicines such as the colour and shape of the tablet/capsule or the appearance of the package.
Editor’s comment
So maybe FDA and others should not be so concerned about tablet size or colour, but should concentrate on informing patients about the benefits of generic medicines, as increased awareness has been shown to increase generics substitution amongst patients and pharmacists [2].
Please feel free to share your thoughts via email to editorial@gabionline.net or in the comments section below. What are your views on generics substitution? Should patients expect generic medicines to look exactly the same as the originator? Would this improve compliance or is price really the crux of the matter?
Related articles
Pakistani immigrants may confuse generics with counterfeit drugs
Generic substitution in Norway
References
1. Heikkilä R, Mäntyselkä P, Ahonen R. Price, familiarity, and availability determine the choice of drug - a population-based survey five years after generic substitution was introduced in Finland. BMC Clinical Pharmacology. 2011;11:20. doi:10.1186/1472-6904-11-20
2. GaBI Online - Generics and Biosimilars Initiative. Generic substitution causing confusion [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 Jan 20]. Available from: www.gabionline.net/Generics/Research/Generic-substitution-causing-confusion
3. Andersen ML et al. How do patients evaluate the newly introduced system of substituting prescriptions? Ugeskr Laeger. 2000;162:6066-9 [in Danish].
4. GaBI Online - Generics and Biosimilars Initiative. FDA to release guidance on generic tablet size [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 Jan 20]. Available from: www.gabionline.net/Guidelines/FDA-to-release-guidance-on-generic-tablet-size








 2
2










Post your comment