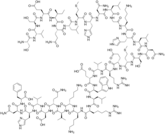
On 26 February 2026, the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) adopted positive opinion, recommending the granting of a marketing authorizatio...
More > 0
0

On 26 February 2026, the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) adopted positive opinion, recommending the granting of a marketing authorizatio...
More >