Biologicals represent many of the most promising new therapies for previously intractable diseases and are becoming increasingly important in the pharmaceuticals market. The global biologicals market in 2020 was worth approximately US$239.2 billion, and in 2020 five of the top 10 best-selling drugs were biologicals compared with a high of seven in 2018, and only three in 2008 [1].
US biosimilars pipeline for supportive care, oncology and TNF inhibitors – 2021
Home/Reports
|
Posted 14/05/2021
 0
Post your comment
0
Post your comment

The biosimilar market in the US is also rapidly growing. In 2019, it was valued at US$737.2 million and was forecast to reach US22,966 million by 2027, a compound annual growth rate of 46.2%. Despite the huge growth in biologicals, there is currently only 19% of the biologicals market, or US$40 billion, already facing some biosimilar competition. In fact, 90% of global biosimilars sales take place in Europe, despite 60% of overall biologicals sales occurring in the US [2].
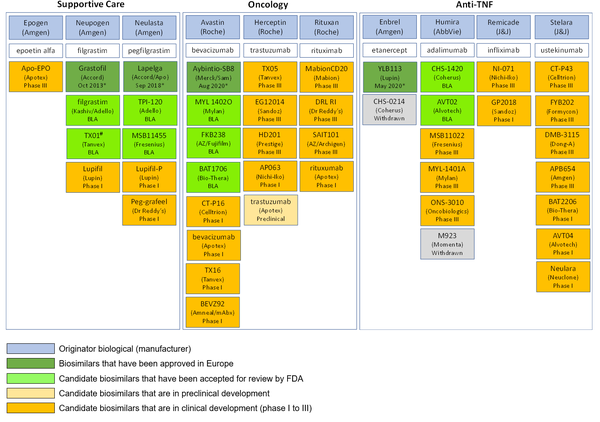
In spite of the lack of uptake of biosimilars in the US, the pipeline for biosimilars still looks healthy. For instance, in supportive care, oncology and tumour necrosis factor (TNF) inhibitors, no fewer than 44 biosimilars are in the pipeline for the US biosimilar marketplace, see Table 1.
In oncology, therapeutic biologicals are used for the prevention and treatment of various types of cancer. However, biologicals are not exclusive to primary treatment, they have also been developed for supportive oncology treatment. Supportive oncological treatment addresses the symptoms that are common with primary chemotherapy.
TNF inhibitors (or anti-TNF drugs) are drugs that help stop inflammation. They are used to treat diseases like rheumatoid arthritis (RA), juvenile arthritis, psoriatic arthritis, plaque psoriasis, ankylosing spondylitis, ulcerative colitis (UC), and Crohn's disease.
Table 1: US pipeline for supportive care, oncology and anti-TNF biosimilars
*Approval date in Europe.
#Rejected by US FDA in September 2019 [3].
Apo: Apotex; AZ: Astra Zeneca; BLA: Biologics License Application; mAb: mAbxience; Sam: Samsung Bioepis; TNF: tumour necrosis factor.
Source: GaBI Online Biosimilars of adalimumab, bevacizumab, epoetin alfa, etanercept, filgrastim, infliximab, pegfilgrastim, rituximab, trastuzumab, ustekinumab.
Related article
US biosimilars pipeline for growth hormone, infertility and bone health drugs – 2021
US biosimilars pipeline for immunosuppressants, insulin and ophthalmology – 2021
Approval and launch dates for US biosimilars – 2021
| LATIN AMERICAN FORUM – Coming soon! To further enhance the objectives of GaBI in sharing information and knowledge that ensure policies supportive of safe biosimilars use, we are pleased to announce that we will be launching a new section on GaBI Online and GaBI Journal, the ‘Latin American Forum’ (in Spanish) featuring the latest news and updates on research and developments in generic and biosimilar medicines in Latin America. Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
LATIN AMERICAN FORUM – Próximamente! Para fomentar los objetivos de GaBI sobre la difusión de información y conocimiento sobre las políticas de apoyo que garantizan el uso seguro de medicamentos biosimilares, nos complace anunciar el lanzamiento de una nueva sección en GaBI Online y GaBI Journal, el ‘Latin American Forum’ (en español), que presentará las últimas noticias y actualizaciones en investigación y desarrollo sobre medicamentos genéricos y biosimilares en Latinoamérica. Regístrese para recibir el boletín informativo GaBI Latin American Forum. Informe a colegas y amigos sobre esta nueva iniciativa. |
References
1. Derbyshire M, Shina S. Patent expiry dates for best-selling biologicals: 2018 update. Generics and Biosimilars Initiative Journal (GaBI Journal). 2019;8(1):24-31. doi:10.5639/gabij.2019.0801.003
2. GaBI Online - Generics and Biosimilars Initiative. The sluggish US biosimilars market [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2021 May 14]. Available from: www.gabionline.net/Reports/The-sluggish-US-biosimilars-market
3. GaBI Online - Generics and Biosimilars Initiative. Biosimilars of filgrastim [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2021 May 14]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-of-filgrastim
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2021 Pro Pharma Communications International. All Rights Reserved.
Source: AmerisourceBergen
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment