At the 20th Biosimilars Medicines Conference held in April 2024, Aurelio Arias, Director of EMEA Thought Leadership at IQVIA, explored the risks and challenges associated with accessing cancer treatments involving biologicals and biosimilars [1].
Role of biologicals and biosimilars in cancer treatment amidst rising cases
Home/Reports
|
Posted 06/08/2024
 0
Post your comment
0
Post your comment
The World Health Organization's (WHO) most recent projections suggest a staggering 77% surge in new cancer cases globally by 2050, compared to the approximate 20 million recorded in 2022 [2].
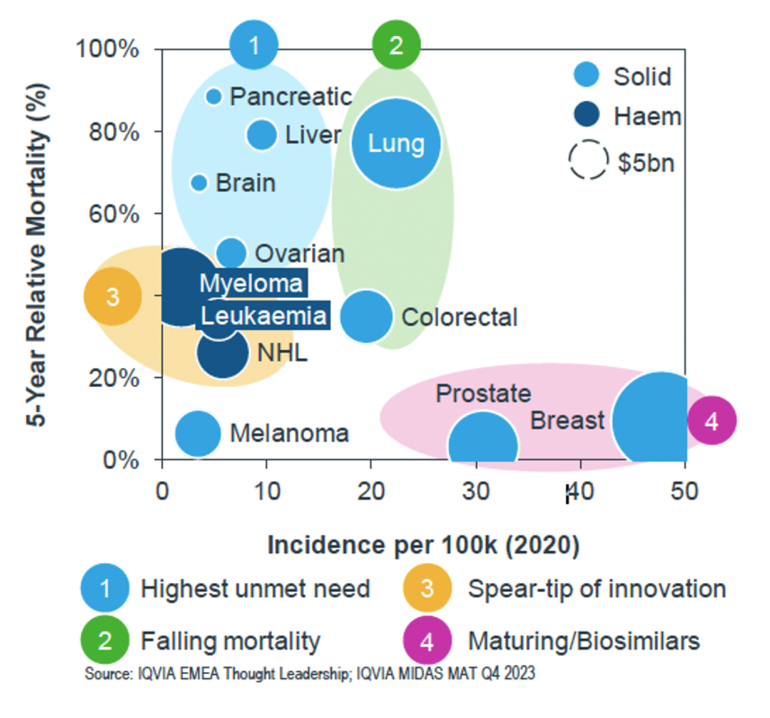
From the 2020 data on cancer incidence per 100,000 person and 5-year relative mortality, see Figure 1, the cancer types (solid tumour) with unmet needs are ovarian, liver, brain and pancreatic cancers. Other solid tumours with the highest incidence are breast and prostate. Lung and colorectal cancers have enjoyed falling mortality rate, with melanoma having the lowest incidence. The good news for breast and prostate cancers is that there are maturing biosimilars for patients, such as trastuzumab and bevacizumab.
Figure 1: Unmet need in oncology (global, US$)
In 2023, the European prescription sales for oncology reached €307 billion, with biologicals accounting for 30%. The growth rate for European oncology, averaging around 14% annually, underscores the rapid advancement and adoption of new therapies. Oncology is and will continue to be Europe's top therapeutic area.
By August 2023, both the European Medicines Agency (EMA) and the US Food and Drug Administration have approved 38 and 22 cancer biosimilars, respectively [2]. These biosimilars have undergone rigorous evaluation and have been deemed comparable to their reference biologic drugs in terms of safety, efficacy, and quality.
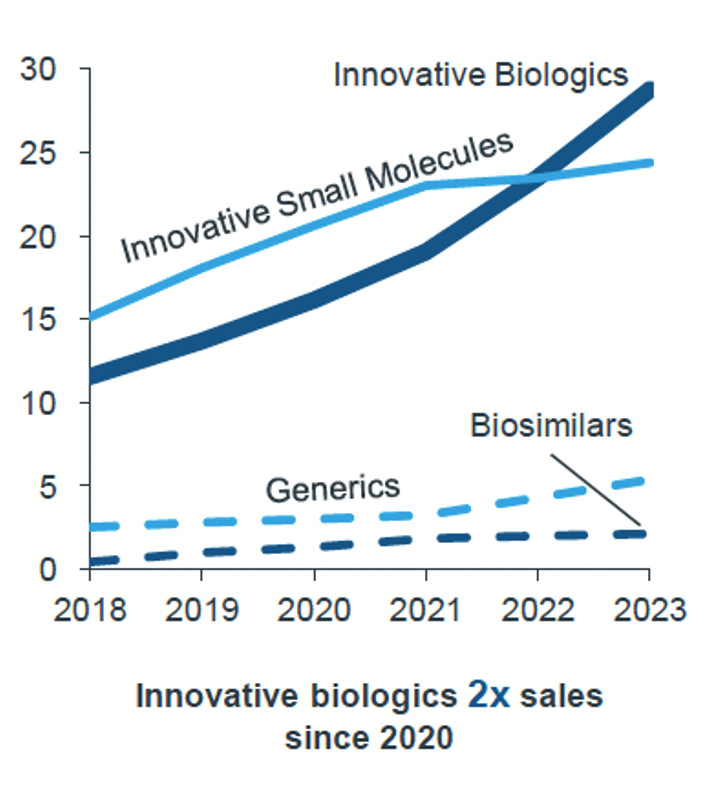
Arias noted that while new medicines are improving outcomes for cancer patients, the sales of innovative biologicals have doubled since 2020, see Figure 2, demonstrating their growing importance and successful market penetration and acceptance. However, this also indicates a growing financial burden on healthcare systems.
Figure 2: Biologicals are a driving force in European oncology Rx sales (2023, Eur bn)
The unmet need in oncology globally (quantified in USD) is substantial, requiring significant investment to address it. However, there is optimism as mortality rates are falling. Additionally, there is a trend of increasing budget allocations for cancer medicines, and the market is maturing with the introduction of biosimilars over the years.
Oncology is and will continue to be Europe's top therapeutic area. In 2023, the EMA has approved 25 new drugs for cancer treatment, four of which are innovative biologicals [3].
Related articles
FDA approves ustekinumab, trastuzumab, and tocilizumab biosimilars
EMA recommends approval of trastuzumab biosimilar Herwenda
|
LATIN AMERICAN FORUM View the latest headline article: Richmond lanzará los biológicos similares bevacizumab Yriviak y adalimumab Armixa Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
FORO LATINOAMERICANO Ver el último artículo de cabecera: Richmond lanzará los biológicos similares bevacizumab Yriviak y adalimumab Armixa !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa. |
References
1. 20th Biosimilars Medicines Conference, April 2024 Medicines for Europe. Future-proofing the Access Roadmap to The Next Wave of Cancer Medicines. Aurelio Arias.
2. GaBI Online - Generics and Biosimilars Initiative. Biosimilars in cancer treatment in Europe and the US [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2024 Aug 6]. Available from: www.gabionline.net/reports/biosimilars-in-cancer-treatment-in-europe-and-the-us
3. GaBI Online - Generics and Biosimilars Initiative. Highlights of EMA approvals in 2023 focus on cancer medicines [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2024 Aug 6]. Available from: www.gabionline.net/reports/highlights-of-ema-approvals-in-2023-focus-on-cancer-medicines
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2024 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment