Although the use of biosimilars for the treatment of autoimmune diseases varies widely by geographical region, the trend is that rheumatologists are prescribing more biosimilars.
Rheumatologists are prescribing more biosimilars
Home/Reports
|
Posted 31/05/2021
 0
Post your comment
0
Post your comment

The data come from two surveys carried out in 2018 and 2020 by GlobalData. The surveys asked high-prescribing rheumatologists from the eight major markets, which included the EU5 (France, Germany, Italy, Spain and the UK), the US, Japan and Australia, about their biosimilar prescription patterns in patients with rheumatoid arthritis (RA).
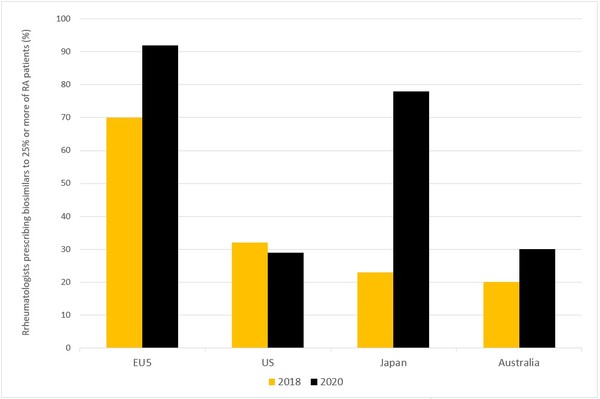
The results point to increased acceptance of biosimilars for the treatment of RA, although at very different rates in the eight markets. Between 2018 and 2020, biosimilar use in the EU5 and Japan increased significantly, while in the US and Australia it stayed at almost the same level, see Figure 1.
Figure 1: Rheumatologists prescribing biosimilars to 25% or more of their RA patients
EU5: France, Germany, Italy, Spain and the UK; RA: rheumatoid arthritis.
Japan saw the largest increase in the two years between the survey. Between 2018 and 2020, the percentage of Japanese physicians using biosimilars in at least 25% of their RA patients jumped from 23% to 78%. Although biosimilar use remains modest in Japan compared to the EU5, increased confidence in biosimilars is likely the reason for the increase in usage. In 2018, 46% of Japanese rheumatologists said they would rather use originator biologicals, citing potential problems with biosimilar manufacturing and doubts about their reliability. In 2020, only 6% of Japanese rheumatologists preferred originator biologicals.
Europe has also seen a significant rise in the use of biosimilars among rheumatologists. In 2020, 92% of rheumatologists from the EU5 reported that they used biosimilars in at least 25% of their RA patients, compared to 70% in 2018. Key factors driving the strong overall uptake of biosimilars in Europe include more liberal interchangeability policies and government-mandated quotas for biosimilar prescription. The introduction of new biosimilars, such as adalimumab, etanercept, infliximab and rituximab, with multiple products at low prices is also thought to have influenced the increased uptake in 2020.
In the US and Australia, however, the use of biosimilars has remained roughly at the same level from 2018 to 2020, with 20%–30% of surveyed rheumatologists reporting that they use biosimilars in 25% or more of their patients.
In the US the reason for the low uptake of biosimilars is likely due to lack of access to biosimilars for the treatment of RA. To date, only infliximab and rituximab biosimilars have been launched in the region, despite adalimumab and etanercept biosimilars having been approved back in 2016 [1]. In addition, infliximab biosimilars in particular have struggled to compete against the originator biological Remicade.
In Australia biosimilars use did grow slightly between 2018 and 2020, from 20% to 30%. However, the level of biosimilar adoption remains low despite having similar regulatory guidelines for biosimilar use as the EU and access to etanercept, infliximab and rituximab biosimilars. One possible reason for this is thought to be physician preference, with 30% of Australian rheumatologists preferring originator biologicals rather than biosimilars. Inexperience with biosimilars, a higher degree of confidence in the quality of originator biologicals and lack of financial incentive to select biosimilars were stated as reasons for this preference.
These survey results point to increased trust in the quality of biosimilars. However, according to GlobalData, the key is now ‘to incentivize physicians to try out the products for the first time or begin to use them more frequently. One key reason for the increased success of biosimilars in the EU5 and Japan is competitive pricing. Until there is a clear financial incentive, it is unlikely the use of biosimilars will increase in the US and Australia without governmental involvement.’
Editor’s comment
Readers interested to learn more about the use of biosimilars in rheumatology are invited to visit www.gabi-journal.net to view the following manuscript published in GaBI Journal:
Use of biosimilars in rheumatology
GaBI Journal is indexed in Embase, Scopus, Emerging Sources Citation Index and more.
Readers interested in contributing a research or perspective paper to GaBI Journal – an independent, peer reviewed academic journal – please send us your submission here.
GaBI Journal Citation Impact
1.7 – CiteScore 2019 (calculated on 6 May 2020)
1.9 – CiteScoreTracker 2020 (Last updated on 6 April 2021)
Submit a manuscript to GaBI Journal
Related articles
Switching to biosimilars in rheumatology
Approval of biosimilars in rheumatology
| LATIN AMERICAN FORUM – Coming soon! To further enhance the objectives of GaBI in sharing information and knowledge that ensure policies supportive of safe biosimilars use, we are pleased to announce that we will be launching a new section on GaBI Online and GaBI Journal, the ‘Latin American Forum’ (in Spanish) featuring the latest news and updates on research and developments in generic and biosimilar medicines in Latin America. Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
LATIN AMERICAN FORUM – Próximamente! Para fomentar los objetivos de GaBI sobre la difusión de información y conocimiento sobre las políticas de apoyo que garantizan el uso seguro de medicamentos biosimilares, nos complace anunciar el lanzamiento de una nueva sección en GaBI Online y GaBI Journal, el ‘Latin American Forum’ (en español), que presentará las últimas noticias y actualizaciones en investigación y desarrollo sobre medicamentos genéricos y biosimilares en Latinoamérica. Regístrese para recibir el boletín informativo GaBI Latin American Forum. Informe a colegas y amigos sobre esta nueva iniciativa. |
Reference
1. GaBI Online - Generics and Biosimilars Initiative. Approval and launch dates for US biosimilars – 2021 [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2021 May 28]. Available from: www.gabionline.net/Reports/Approval-and-launch-dates-for-US-biosimilars-2021
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2021 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment