The market for copy biologicals in China has significantly grown over the past decade. The government has been actively promoting the development and use of copy biologicals as a way to improve access to affordable health care. As a result, the number of pharmaceutical companies developing copy biologicals has also increased rapidly and is expected to continue to grow in the coming years.
Pharmaceutical companies in China manufacturing copy biologicals
Home/Reports
|
Posted 20/06/2023
 0
Post your comment
0
Post your comment
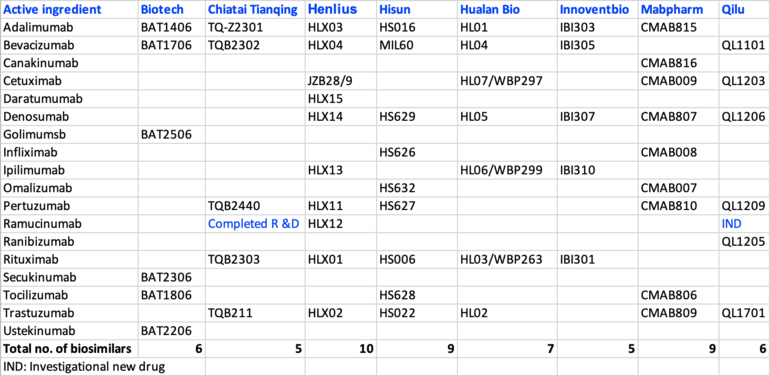
China has over 60 pharmaceutical companies involved in the development of copy biologicals products. Representative companies include Biotech, Chiatai Tianqing, Henlius, Hisun, Hualan Bio, Innoventbio, Mabpharm and Qilu Pharmaceutical. Table 1 shows the list of biosimilar monoclonal antibodies (mAbs) of these companies. Henlius and Innoventbio have the most biosimilar products on the market, however, Innoventbio has fewer biosimilars than other companies [1].
Table 1: Copy biologicals products and their representative companies in China
In the US and the European Union (EU), the development of biosimilar products is equally rapid. In terms of biosimilar mAbs, a large number of biosimilar products were approved by US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) [2-3]. The biosimilar markets in the EU and the US are more competitive and energetic than that of China.
In China, the regulatory body for the approval of medicines, including biologicals, is the National Medical Products Administration (NMPA), formerly the China Food and Drug Administration (CFDA) [4].
FDA and NMPA have similar requirements in the development of biosimilars. Comparative data evaluated from a systematic process consisting of pharmaceutical studies, non-clinical studies (animal studies) and clinical studies should be provided by the manufacturer of a proposed biosimilar. FDA and NMPA both allow biosimilars to be approved for indications without direct studies, but extrapolation is not automatic. NMPA requires simultaneous fulfilment of certain conditions for indication extrapolation.
As one of the fastest-growing markets for biosimilars due to its large population and increasing demand for affordable biological drugs, Chinese government has introduced new regulations that provide stronger patent protections for biosimilar mAbs.
Editor’s comment
European Medicines Agency regulatory requirements ensure the same high standards of quality, safety and efficacy for biosimilars as for originator biologicals, and also include a rigorous comparability exercise with the reference product but they are not universally accepted by regulatory bodies outside of the European Union (EU). It should be noted that copy biologicals approved in China might not have been authorized if they had been subjected to the strict regulatory processes required for approval of biosimilars in the EU.
Related articles
Biosimilar monoclonal antibodies in China
Overview of monoclonal antibody biosimilars in Latin America
Biosimilar mAb development – quality similarity considerations
Mabpharm gains approval for infliximab biobetter in China
|
LATIN AMERICAN FORUM View the latest headline article: AMLAC: agencia reguladora de medicamentos de América Latina y el Caribe establecida Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
FORO LATINOAMERICANO Ver el último artículo de cabecera: AMLAC: agencia reguladora de medicamentos de América Latina y el Caribe establecida !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa. |
References
1. Liu J-W, Yang Y-H, Wu N, Wei J-F. Biosimilar monoclonal antibodies in China: a patent review. Bioengineered. 2022;13(6):14503-18.
2. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in the US. [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2023 Jun 20]. Available from:
www.gabionline.net/biosimilars/general/biosimilars-approved-in-the-us
3. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in Europe. [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2023 Jun 20]. Available from:
www.gabionline.net/biosimilars/general/biosimilars-approved-in-europe
4. GaBI Online - Generics and Biosimilars Initiative. Copy biologicals approved in China [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2023 Jun 20]. Available from: www.gabionline.net/biosimilars/general/copy-biologicals-approved-in-china
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2023 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment