At the online session of BIOS 22, Biosimilar medicines: changing patient care pathways, changing outcomes, speaker Nadia Amer, Health Economist Analyst at the Health Product Department in the Caisse Nationale d’Assurance Maladie (CNAM) in France, delivered a presentation based on concrete examples and experiences of biosimilar medicines in France [1].
Key figures of biosimilar medicines in France
Home/Reports
|
Posted 30/09/2022
 0
Post your comment
0
Post your comment

Her presentation included some key figures about the biosimilar market in France. Biosimilars turnover is growing very rapidly. It had been multiplied by 5.6 times over the past five years and it reached Euros 1.6 billion in 2021. More than half of turnover is generated by hospital (54%) and community pharmacies (46%). Biosimilars represent almost half (48%) of the biological market and a 3% of the total pharmaceutical market.
However, and despite these growing numbers, the penetration rate is quite low with 25% on average measured as volume share of drugs delivered in the community whether prescribed by hospital- or community-based practitioners, see Figure 1,. This is far from the objective of 80% that was to be achieved by 2023.
Figure 1: Heterogenous penetration rate (volume share, community)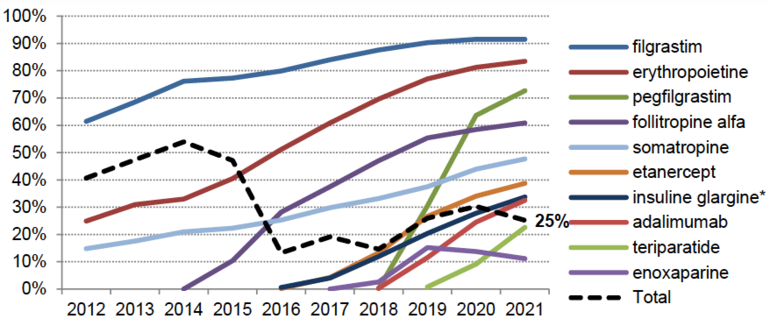
In a series of the next two articles, the incentives and challenges of biosimilar medicines in France will be discussed.
Related articles
Challenges of biosimilar medicines in France
Incentives promoting use of biosimilar medicines in France
Competition from biosimilars drives price reductions for biologicals in France
The cost-effectiveness of biosimilars
| LATIN AMERICAN FORUM The new section of the ‘Latin American Forum’ on GaBI has been launched. The objective of this new section is to provide you with all the latest news and updates on developments of generic and biosimilar medicines in Latin America in Spanish. View the latest headline article: La EMA pide la intercambiabilidad de los biosimilares en toda la UE Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative. FORO LATINOAMERICANO Se ha lanzado la nueva sección del ‘Foro Latinoamericano’ sobre GaBI. El objetivo de esta nueva sección es brindarle las últimas noticias y actualizaciones sobre desarrollos de medicamentos genéricos y biosimilares en América Latina en español. Ver el último artículo de cabecera: La EMA pide la intercambiabilidad de los biosimilares en toda la UE !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa. |
Reference
1. Biosimilar medicines: changing patient care pathways, changing outcomes. Webinar BIOS 22. 28 April 2022, Thursday. Speaker: Nadia Amer, Health Economics Analyst, Health Product Department, Caisse Nationale d’Assurance Maladie (CNAM), France.
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2022 Pro Pharma Communications International. All Rights Reserved.
Source: CNAM 2021 data
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment