Biosimilar monoclonal antibodies (mAbs) are currently a major focus of research and development in China with policies support. mAbs have become crucial therapeutics for treating diseases such as oncology and autoimmune diseases. As competition from biosimilars increases in China, the price of mAbs is decreasing and access to them is increasing.
Biosimilar monoclonal antibodies in China
Home/Reports
|
Posted 10/02/2023
 0
Post your comment
0
Post your comment

A review carried out by Liu et al. presents the biosimilar monoclonal antibodies market and development status as well as approval process in China and analyses the patents in this field [1].
To do so, the review highlights on a summary of the patents related to biosimilar mAbs in China and their advantages, gives an overview of Chinese biosimilar monoclonal antibody market and development and assess patents that improve the prescription of preparations account for the majority.
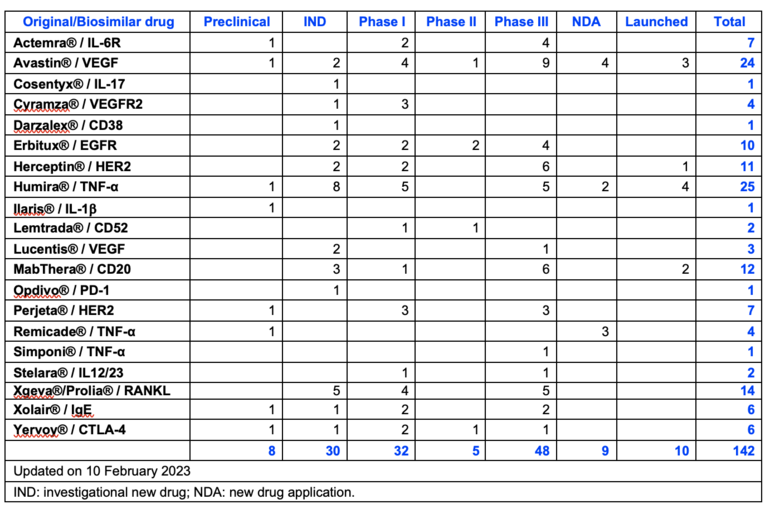
Since the first copy biological Rituximab injection by Henlius was launched in 2019, 142 biosimilar mAbs involved 16 targets have been researched and developed in China, and 10 of them have been launched, see Table 1. Adalimumab and bevacizumab are the hotspots in the Chinese biosimilar market, while mAbs with new targets show less competition.
Table 1: R & D projects of different biosimilars conducted in China
The 10 biosimilar mAbs launched in China are Avastin (bevacizumab) x 3, Herceptin (trastuzumab) x 1, Humira (adalimumab) x 4, and MabThera (rituximab) x 2. China has over 60 pharmaceutical companies involved in the development of biosimilar products.
The following series of two articles will provide a summary of the representative companies for biosimilar products and the patents of biosimilar mAbs in China.
Related articles
Pharmaceutical companies in China manufacturing copy biologicals
Mabpharm gains approval for infliximab biobetter in China
Copy biologicals approved in China
|
LATIN AMERICAN FORUM View the latest headline article: DARS: avance el desarrollo de biosimilares en los EE. UU Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
FORO LATINOAMERICANO Ver el último artículo de cabecera: DARS: avance el desarrollo de biosimilares en los EE. UU !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa. |
Reference
1. Liu J-W, Yang Y-H, Wu N, et al. Biosimilar monoclonal antibodies in China: a patent review, bioengineered. 2022;13(6):14503-18. doi:10.1080/21655979.2022.2090206
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2023 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment