In a presentation given by Doug Long, the expected launches and increase in uptake of biosimilars for the US market were discussed.
Biosimilar launches and uptake expected to increase in the US
Home/Reports
|
Posted 22/10/2021
 0
Post your comment
0
Post your comment

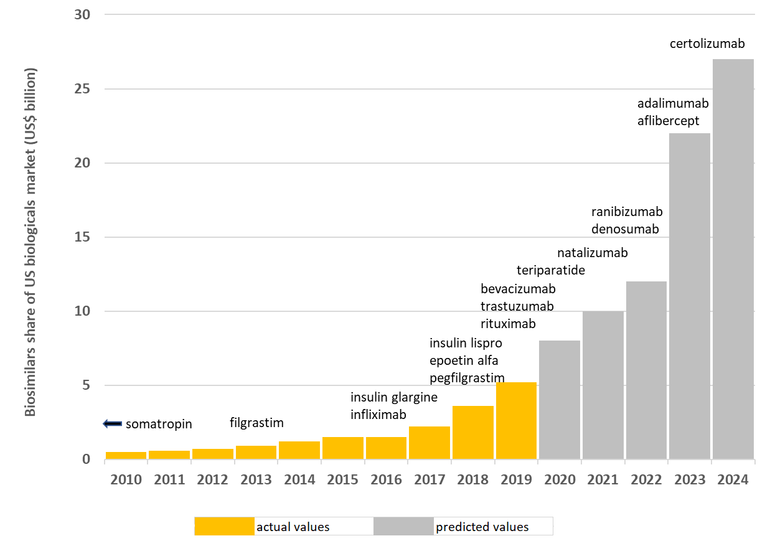
The presentation was based on the report entitled ‘Biosimilars in the US 2020–2024: Competition, savings and sustainability’ by IQVIA, it outlined how expected biosimilar launches and uptake are likely to increase overall spending on biosimilars significantly to US$16–US36 billion by 2024, see Figure 1.
In the past, the US biosimilars market has been described as ‘sluggish’ [2]. Barriers to market-entry for biosimilars include patent litigation and thickets, anti-competitive settlements and the length of time from US Food and Drug Administration (FDA) approval to market launch [3]. In fact, biosimilars currently make up only 2.3% of the US biologicals marketplace. Currently, 90% of global biosimilars sales take place in Europe, despite 60% of overall biologicals sales occurring in the US [2].
Figure 1: Expected biosimilar launches and uptake as a share of the US biologicals market
Source: Biosimilars in the US: Competition, savings and sustainability. Report by the IQVIA Institute for Human Data Science.
The landscape does, however, appear to be changing. Recent biosimilars have achieved high volume shares and IQVIA projects that these will reach more than 50% within the first two years of launch. For example, bevacizumab and trastuzumab biosimilars, which were both launched in the US in July 2019 achieved 42% and 38% market share, respectively, 12 months after first introduction. This compares to infliximab, pegfilgrastim and filgrastim biosimilars, which were launched in November 2016, July 2018 and November 2013, respectively, and only achieved 6%, 28% and 39% two years after launch, respectively.
Biosimilars launched to date account for 20% of competitive molecule volume, but this is expected to increase. The analysis by IQVIA projects savings over the next five years (2020–2024) as a result of biosimilars to exceed US$100 billion. This compares to only US$18 billion in the 2010–2018 period and US$19 billion in the 2015–2019 period.
Related articles
Biosimilars approved, launched or in development in the US
US savings from biosimilars could exceed US$100 billion
| LATIN AMERICAN FORUM The new section of the ‘Latin American Forum’ on GaBI has been launched. The objective of this new section is to provide you with all the latest news and updates on developments of generic and biosimilar medicines in Latin America in Spanish. View this week’s headline article: Grupos de gastroenterólogos argentinos emiten su posicionamiento sobre el uso de biosimilares Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative. LATIN AMERICAN FORUM Se ha lanzado la nueva sección del ‘Foro Latinoamericano’ sobre GaBI. El objetivo de esta nueva sección es brindarle las últimas noticias y actualizaciones sobre desarrollos de medicamentos genéricos y biosimilares en América Latina en español. Vea el artículo principal de esta semana: Grupos de gastroenterólogos argentinos emiten su posicionamiento sobre el uso de biosimilares Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa. |
References
1. IQVIA. Biosimilars in the US 2020-2024: Competition, savings and sustainability. Report by the IQVIA Institute for Human Data Science. 29 September 2020.
2. GaBI Online - Generics and Biosimilars Initiative. The sluggish US biosimilars market [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2021 Oct 22]. Available from: www.gabionline.net/reports/The-sluggish-US-biosimilars-market
3. GaBI Online - Generics and Biosimilars Initiative. Overcoming hurdles to biosimilars cost savings in the US [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2021 Oct 22]. Available from: www.gabionline.net/biosimilars/research/Overcoming-hurdles-to-biosimilars-cost-savings-in-the-US
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2021 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment