A study on the estimation of patients with rheumatoid arthritis (RA) as well as those patients who are potentially eligible for the treatment of the disease with biological drugs, was presented by Luca Degli Esposti of CliCon Società Benefit Italy at the 18th Biosimilar Medicines Conference in October 2022.
Biological therapies in patients with rheumatoid arthritis
Home/Reports
|
Posted 03/02/2023
 0
Post your comment
0
Post your comment
Inclusion criteria for the study
RA patients were identified by the presence of the following criteria, which define the presence of the diagnosis:
– Rheumatoid arthritis exemption (code 006), or
– Patients who have undergone at least one hospitalization for rheumatoid arthritis in which the ICD-9CM714 code is indicated at each level among the discharge diagnoses throughout the period of availability of data within the databases (2013‒2017) [1].
The prevalence of RA in 2017 was 0.52% in the overall population. In the Italian context, the prevalence was approximately estimated at 0.41%‒0.48% [2].
In order to make an estimation of patients potentially eligible for treatment with biological drugs, the eligibility criteria to biological therapies, see Table 1, and the distribution of patients by those eligibility criteria were analysed [1].
| Table 1: Eligibility criteria to biological therapies |
| A. MTX treatment failure |
Use of MTX for at least six months, then switch to a different csDMARD Failure of MTX therapy (ATC code: L01BA01) lasting at least six months and initiation of treatment with a second csDMARD |
| B. corticosteroids ≥7.5 mg/die |
Corticosteroid treatment for at least six months, with a dose ≥7.5mg/die Treatment for at least 6 months with corticosteroid (ATC code: H02) at a dosage of at least 7.5mg/day |
| c. MTX contraindication |
Contraindication to MTX therapy, defined as patients on therapy or hospitalized for renal damage, interstitial lung disease or hepatic failure Presence of treatment or hospitalization for kidney injury (ICD-9-CM codes: 580-589), pulmonary interstitial disease(ICD-9-CM codes: 510-519) or liver injury (ICD-9-CM codes: 570-573) |
| RA: rheumatoid arthritis; csDMARDs: conventional synthetic disease-modifying antirheumatic drugs; MTX: methotrexate. |
Distribution of patients by eligibility criteria:
Among patients identified as potentially eligible to biological therapy:
– 0.7% (1,896) fulfilled eligibility criteria A, that is, it had at least one treatment failure with MTX
– 5.8% (15,833) of patients instead were eligible according to criterion B, that is, in treatment with corticosteroid ≥7.5 mg/day for at least 6 months
– 2.8% (7,788) of patients met criterion C, that is contraindication for MTX
– 107 patients presented the criteria A+B; 53 criteria A+C, and 810 criteria B+C.
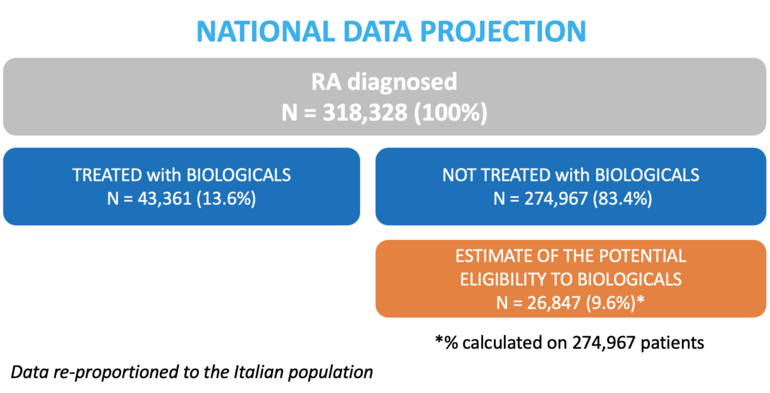
As shown in Figure 1, data re-proportioned to the Italian population in 2017 estimated in this analysis a total of 318,328 patients affected by RA. According to the estimation, 43,361 (13.6%) of them were treated with biotechnological treatments, while 274,967 (83.4%) were not prescribed with these drugs. Among the latter, 26,487 (9.6%) patients were estimated to be eligible for biotechnological therapy.
Figure 1: Flowchart of the study population
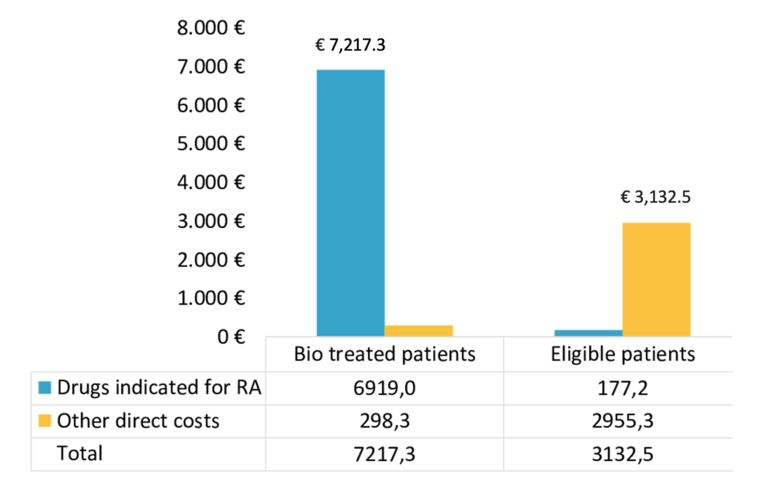
The mean total annual healthcare cost, see Table 2, for RA patients treated with biotechnological therapy was estimated to be €7,217.3; of which €6,919.0 for drugs indicated for RA and €298.3 for other direct costs. For patients eligible for such therapy, the estimated total annual expenditure was €3,132.5, of which €177.2 for drugs indicated for RA and €2,955.3 for other direct costs.
Table 2: Mean annual costs for patients treated with biotechnological therapy and eligible for biotechnological therapy
Information on the number of patients with inflammatory bowel diseases and psoriasis that are possibly eligible for biological treatments and are still not receiving those treatments were presented at the 18th Biosimilar Medicines Conference in October 2022.
Related article
Estimation of patients potentially eligible to biological therapies
|
LATIN AMERICAN FORUM View the latest headline article: EE.UU. frente a Alemania y Suiza: El mercado estadounidense de biosimilares se queda atrás con precios más altos Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
FORO LATINOAMERICANO Ver el último artículo de cabecera: EE.UU. frente a Alemania y Suiza: El mercado estadounidense de biosimilares se queda atrás con precios más altos !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa. |
References
1. Degli Esposti L, Perrone V, Sangiorgi D, et al. Assessment of patients affected by rheumatoid arthritis eligible for biotechnological agents and evaluation of their healthcare resource utilization and related costs. Reumatismo. 2021;73(1):5-14.
2. Rossini M, Rossi E, Bernardi D, et al. Prevalence and incidence of rheumatoid arthritis in Italy. Rheumatol Int. 2014;34(5):659-64.
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2023 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment