Dr Yow-Ming Wang provided welcoming remarks during the biosimilars webinar hosted by the University of Maryland Center of Excellence in Regulatory Science and Innovation (M-CERSI) and the U.S. Food and Drug Administration (FDA).
Approaches to streamline biosimilar interchangeable programmes
Home/Reports
|
Posted 03/06/2022
 0
Post your comment
0
Post your comment

In a joint webinar entitled: Biosimilars: a decade of experience and future directions, hosted by the M-CERSI and FDA in April 2022, Dr Yow-Ming Wang, Associate Director for Biosimilars and Therapeutic Biologics; Office of Clinical Pharmacology of FDA, delivered the opening remarks for the event.
Dr Wang stated that a biosimilar product is highly similar to and has no clinically meaningful differences in terms of safety, purity, and potency, i.e. safety and effectiveness, from an FDA-approved reference product.
An interchangeable product is a biosimilar to the reference product and can be expected to produce the same clinical result as the reference product in any given patient.
It has been proven that there is no greater risk in terms of safety or diminished efficacy when switching or alternating a reference product by a biosimilar product.
Back in May 2019, FDA has issued the final guidance on the pathway for interchangeable biologicals [1].
By April 2022, FDA has approved 34 biosimilar products and two of them are interchangeable products, see Figure 1. The two biosimilars that have interchangeable product status are Humira and Lantus.
Figure 1: FDA approved biosimilar products and their data requirements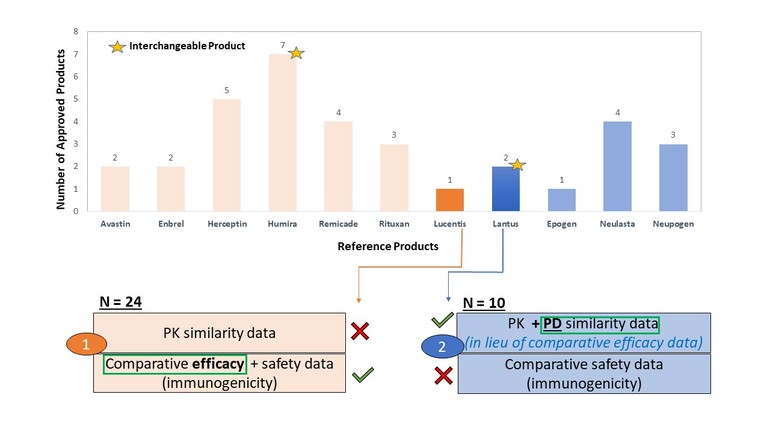
Figure 1 shows the number of biosimilars approved in the US by the reference product. The colour code indicates the clinical data used to support their approval.
a) Group 1/orange colour: Pharmacokinetic (PK) similarity data and comparative efficacy + safety data (immunogenicity)
b) Group 2/blue colour: PK + pharmacodynamic (PD) similarity data and comparative safety data (immunogenicity)
By comparison, PK similarity data for biosimilars to Lucentis as well as comparative immunogenicity data for biosimilars to Lantus were deemed not necessary for their approval.
According to Dr Wang, over the years, FDA learned two approaches to streamline biosimilar into interchangeable programmes, and these are:
1. Adopt the approach of PK + PD similarity studies
Benefits of PK and PD studies over comparative clinical studies:
– Smaller sample size (higher sensitivity with PD endpoints vs clinical endpoints)
– Shorter study duration
– Ease of recruitment when feasible in healthy subjects
See the FDA guidance [2].
2. Certain studies are not necessary when scientifically justified
For example, comparative immunogenicity data for insulin products may not be needed.
See the FDA guidance [3].
Given that, more scientific innovations through collaboration with all stakeholders are needed.
However, biosimilar interchangeability is a topic with many contrasting views and has been always highly debated [4].
Since the regulatory pathway of biosimilars was available in 2010, FDA has invested in various initiatives to advance biosimilars and interchangeable products. To continue with this effort during the webinar were discussed the ways to increase efficiency in biosimilar development such as increasing the use of PK+PD approach, as well as ways to improve biosimilar adoption.
Related articles
FDA accepts application for interchangeability of adalimumab biosimilar Abrilada
Are systematic switch studies for biosimilars necessary?
The US needs to learn from Europe to increasing access to biosimilars
| LATIN AMERICAN FORUM The new section of the ‘Latin American Forum’ on GaBI has been launched. The objective of this new section is to provide you with all the latest news and updates on developments of generic and biosimilar medicines in Latin America in Spanish. View last week’s headline article: Nomenclature of biologicals and biosimilars in Peru Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative. LATIN AMERICAN FORUM Se ha lanzado la nueva sección del ‘Foro Latinoamericano’ sobre GaBI. El objetivo de esta nueva sección es brindarle las últimas noticias y actualizaciones sobre desarrollos de medicamentos genéricos y biosimilares en América Latina en español. Vea el artículo principal de la semana pasada: Nomenclature of biologicals and biosimilars in Peru !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa. |
References
1. GaBI Online - Generics and Biosimilars Initiative. FDA-issues-final-guidance-on-interchangeable-biologicals [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2022 May 27]. Available from: www.gabionline.net/guidelines/FDA-issues-final-guidance-on-interchangeable-biologicals
2. U.S. Food and Drug Administration. Clinical Pharmacology Data to Support a Demonstration of Biosimilarity to a Reference Product. December 2016 [homepage on the Internet]. [cited 2022 May 27]. Available from: www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-pharmacology-data-support-demonstration-biosimilarity-reference-product
3. U.S. Food and Drug Administration. Clinical immunogenicity considerations for biosimilar and interchangeable insulin products. November 2019
[homepage on the Internet]. [cited 2022 May 27]. Available from: www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-immunogenicity-considerations-biosimilar-and-interchangeable-insulin-products
4. GaBI Online - Generics and Biosimilars Initiative. Will the unclear path of biosimilar interchangeability become clearer? [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2022 May 27] Available from:
www.gabionline.net/biosimilars/research/will-the-unclear-path-of-biosimilar-interchangeability-become-clearer
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2022 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment