Drug products on the shortages list in the US are concentrated into a small number of disease areas, according to a new report published by IMS Institute for Healthcare Informatics (IMS Institute) [1]. The majority are generic injectables, in fact half of all generic injectables on the US market are on the shortages list, and 16% of drug shortages involve life-saving cancer drugs. This means that the patients most affected by the shortages are mostly acute-care patients being treated in hospitals and out-patient facilities.
Which drugs are affected by drug shortages in the US
Home/Reports
|
Posted 27/01/2012
 0
Post your comment
0
Post your comment

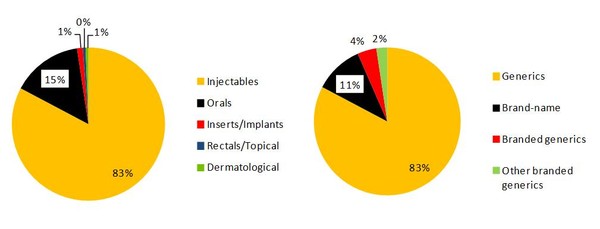
Of the 168 products on the FDA drug shortage list as of 7 October 2011, the majority (139 out of 168 or 83%) were sterile injectables and 83% were generics, see Figure 1. Brand-name drugs accounted for only 11% of the shortages and 4% were branded generics.
Figure 1: Proportion of drugs by type contributing to drug shortages
Source: IMS Institute for Healthcare Informatics [1]
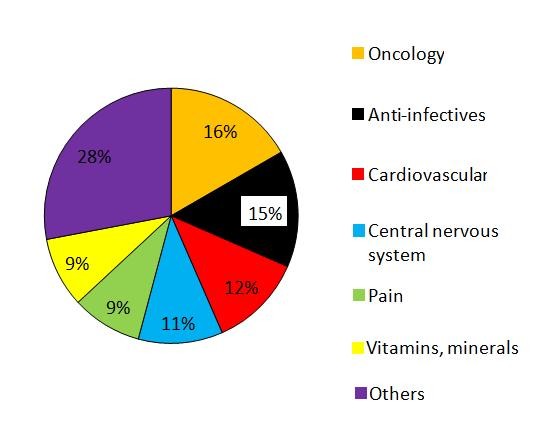
Five therapy areas accounted for 63% of the drugs on the shortages list, see Figure 2. Cancer drugs were the therapy area most affected by the shortages, with 28 of the 168 shortages affecting cancer drugs (16%). Anti-infectives was the second largest group with 15% of shortages being anti-infectives. These were followed by cardiovascular drugs (12%), central nervous system (11%), pain medications, and vitamins and minerals (both 9%).
Figure 2: Proportion of drugs by therapy area contributing to drug shortages
Source: IMS Institute for Healthcare Informatics [1]
Of the 28 cancer medications on the drug shortages list, 22 are used in chemotherapy treatment of cancer, of which 20 are injectable. For these injectable oncology products, an estimated 550,000 patients received treatments during the year ending 30 June 2011.
The shortage problem appears to be not just a problem for older drugs, which are often less profitable, as has been previously reported [2]. Although half of the drugs on the drug shortages list came to the market before 1990, a further 25% were introduced to the market since 2000.
Related articles
Early warning system for drug shortages in the US
Volume and sales of drugs on the shortages list in the US
Suppliers of products on the drug shortages list in the US
Investigating drug shortages in the US
References
1. IMS Institute for Healthcare Informatics. Drug Shortages: A closer look at products, suppliers and volume volatility. November 2011.
2. GaBI Online - Generics and Biosimilars Initiative. US drug shortages – frustration and safety concerns [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 Jan 27]. Available from: www.gabionline.net/Reports/US-drug-shortages-frustration-and-safety-concerns
Source: IMS
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment