In Europe, the use of biosimilars exhibits different rates in different countries. This can lead to inequalities in access to biologicals, for instance, for the treatment of autoimmune diseases such as arthritis [1].
Use of biosimilars in Europe differs across countries
Home/Reports
|
Posted 08/08/2014
 0
Post your comment
0
Post your comment
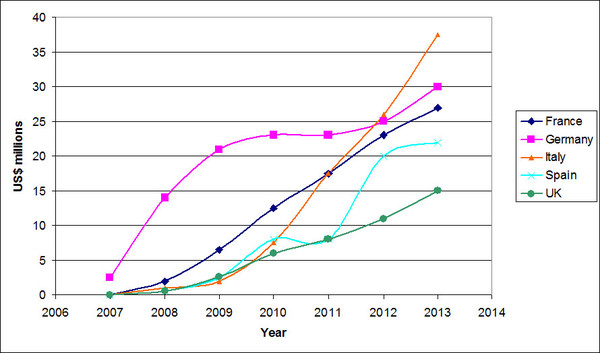
Germany is still leading the way in its fast uptake of biosimilars, while France and the UK exhibit consistent uptake of biosimilars after launch. In fact, Germany has the highest use of biosimilars in Europe, with around 50% volume uptake [2]. However, despite initial cultural resistance, Italy and Spain are now catching up and increasing the uptake of biosimilars in the countries, see Figure 1 [3].
Figure 1: Biosimilar sales across the EU5
Source: IMS Health, MIDAS, MAT Dec 2013
The biosimilars market in the European Union 5 (EU5: France, Germany, Italy, Spain and UK) now stands at US$490 million, a massive increase since 2007 when the market for biosimilars had barely taken off. Filgrastim biosimilars are leading the way when it comes to market penetration, and almost resemble generic drugs in some countries, reaching market shares of 60–80% across the EU5.
Related articles
Biologicals dominate Europe’s best sellers
Factors supporting a sustainable European biosimilars market
Germany wants to increase biosimilars penetration
References
1. GaBI Online - Generics and Biosimilars Initiative. Inequality in Europe over access to biologicals for arthritis [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2014 Aug 8]. Available from: www.gabionline.net/Reports/Inequality-in-Europe-over-access-to-biologicals-for-arthritis
2. GaBI Online - Generics and Biosimilars Initiative. UK biosimilars uptake lower than in some other EU countries [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2014 Aug 8]. Available from: www.gabionline.net/Reports/UK-biosimilars-uptake-lower-than-in-some-other-EU-countries
3. Sheppard A. Biological/biotechnological and biosimilars market: the global outlook with special focus on Europe. 12th EGA International Biosimilar Medicines Conference; 3–4 April 2014; London, UK.
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2014 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment