The uptake of biosimilars in different countries was a subject discussed by both Dr Jaclyn Bosco and Mr Murray Aitken of IQVIA.
Uptake of biosimilars in different countries varies
Home/Reports
|
Posted 08/11/2019
 0
Post your comment
0
Post your comment

Dr Bosco gave her presentation on ‘Biosimilar considerations for real world research and stakeholder questions’ and Mr Murray gave his presentation on ‘Biosimilars in practice: a global perspective’ at the Drug Information Association’s (DIA) Biosimilars Conference, which was held on 23‒24 September 2019 in Bethesda, Maryland, USA [1, 2].
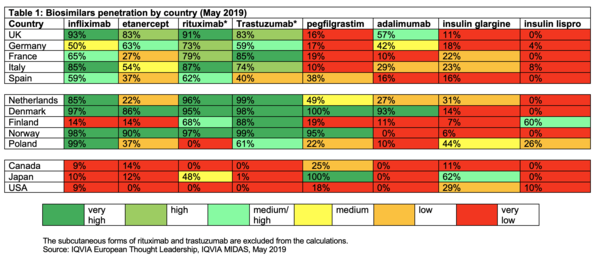
Despite the fact that biologicals have had a profound impact on health care, and the fact that biosimilars have the potential to increase access to these often life-changing products, there is varying uptake across different countries and regions, see Table 1.
Such differences in uptake from country to country can lead to inequalities in access to biologicals, for instance, for the treatment of autoimmune diseases such as arthritis [3].
Reasons for the slower than expected uptake in Europe include the lack of automatic substitution of biosimilars for originator products, and the fact that many doctors and patients are reluctant to switch or substitute, given their lack of familiarity with biosimilars [4].
Related articles
Designing fit-for-purpose biosimilar studies
Considerations for real-world research on biosimilars
Impact of biologicals on health care
References
1. Bosco J. Biosimilar considerations for real world research and stakeholder questions. DIA Biosimilars Conference; 2019 Sep 23‒24; Bethesda, Maryland, USA.
2. Aitken M. Biosimilars in practice: a global perspective. DIA Biosimilars Conference; 2019 Sep 23‒24; Bethesda, Maryland, USA.
3. GaBI Online – Generics and Biosimilars Initiative. Use of biosimilars in Europe differs across countries [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Nov 8]. Available from: www.gabionline.net/Reports/Use-of-biosimilars-in-Europe-differs-across-countries
4. Felix AE, Gupta A, Cohen JP. Barriers to market uptake of biosimilars in the US. Generics and Biosimilars Initiative Journal (GaBI Journal). 2014;3(3):108-15. doi:10.5639/gabij.2014.0303.028
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2019 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment