This article provides an overview of the biosimilars currently available in oncology supportive care, nephrology/oncology supportive care and inflammation in the US.
US market trends in oncology/nephrology supportive care and inflammation biosimilars
Home/Reports
|
Posted 11/12/2020
 0
Post your comment
0
Post your comment
Oncology supportive care
Oncology supportive care is the most mature US biosimilar category. US Food and Drug Administration (FDA) approved Granix in 2012, though not under the pathway created by the Biologics Price Competition and Innovation Act of 2009 (BPCI Act). Zarxio was the first biosimilar approved under the 351(k) pathway (in 2015), and also the first biosimilar to become commercially available (also in 2015).
The biosimilars available for oncology supportive care consist of pegfilgrastim, filgrastim and epoetin alfa.
Pegfilgrastim
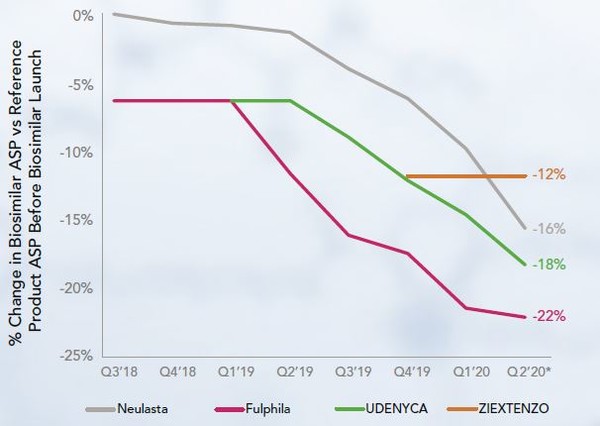
The pegfilgrastim originator is Neulasta. All three biosimilars of pegfilgrastim were launched with a wholesale acquisition cost (WAC) and acquisition sales price (ASP) below that of Neulasta. The ASP of all pegfilgrastim products, including the reference, have declined since the launch of the first biosimilar, see Figure 1.
Figure 1: ASP of pegfilgrastim products at biosimilars launches
*Q2’20 sales data through July 3, 2020; monthly rollup based on 4-4-5 calendar.
Biosimilar WAC price used for comparing against reference product ASP until biosimilar ASP is available.
ASP: average sales price.
Source: Analysource.
Pegfilgrastim biosimilars now account for nearly 30% of sales by volume. The uptake trends of other biosimilars (as described in [1]) show the first biosimilar capturing the majority biosimilars market share. In contrast, with pegfilgrastim, it is the second to launch, Udencia, that held the largest biosimilar market share (21% of total market) when this market trend report was compiled. The first to launch, Fulphila, held just 6%, Ziextenzo launched in late 2019 and held 1%.
Fulphila, Ziextenzo and Udenyca were approved in the US in June 2018 [2], November 2019 [3] and October 2016 [4], respectively. A recent Nyvepria (pegfilgrastim-apgf) has been approved in June 2020 [5]
Filgrastim
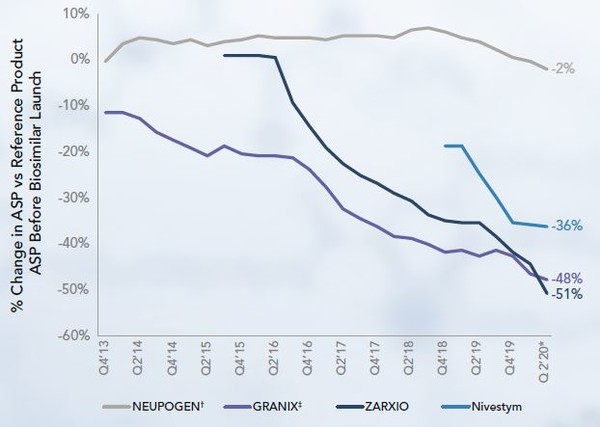
Th filgrastim originator is Neupogen. Two filgrastim biosimilars have launched since 2015, and both with a lower WAC and ASP than Neupogen. In addition, in 2013, Granix was launched which is not a biosimilar of filgrastim as it was approved with a Food and Drugs Association (FDA) Biologics License Application prior to the enactment of the biosimilar approval pathway. Granix launched at lower WAC and ASP than the originator, but the first biosimilar to launch, Zarxio, launched at WAC and ASP lower than Neupogen, but higher than Granix. The second biosimilar to launch, Nivestym, launched at WAC below all its predecessors. However, its ASP was below the originator but above the other competitor products at time of launch, see Figure 2.
Figure 2: ASP of filgrastim products at biosimilars launches
*Q2’20 sales data through July 3, 2020; monthly rollup based on 4-4-5 calendar.
†NEUPOGEN®’s biosimilar price-response strategy focused on account-level provider contracting.
This targeted approach modestly increased the ASP-eligible discount rate, resulting in a more stable ASP trend.
‡GRANIX is not a biosimilar. It was approved under a full Biologics License Application, which was
submitted to the FDA before enactment of the biosimilar approval pathway.
Biosimilar WAC price used for comparing against reference product ASP until biosimilar ASP is available.
ASP: average sales price.
Since the launch of Granix in 2013 its ASP has decreased by 51%. The two biosimilars also reduced significantly by the first quarter of 2020, whereas the ASP of Neupogen has remained relatively stable.
Filgrastim biosimilars uptake has been high and in early 2020, Neupogen held a market share of just 28%, whereas the biosimilar Zarxio held 48%.
Zarxio (filgrastim-sndz) was the first product approved in the US as a biosimilar in 2015 [6], and Nivestym (filgrastim-aafi) was approved in the US in July 2018 [7].
Nephrology/oncology supportive care
Epoetin alfa
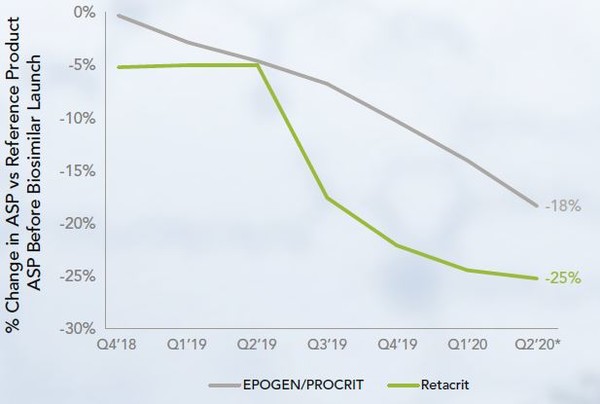
There are two epoetin alfa reference products, Epogen and Procrit. These are the same molecule, marketed by different companies in separate therapeutic areas. They have different WACs but the same ASP. There has been the launch of one biosimilar, Retacrit, at a lower WAC and ASP than both references. Since the biosimilar’s launch, both its ASP and that of the reference products have decreased significantly, see Figure 3.
Figure 3: ASP of epoetin alfa products at biosimilars launch
*Q2’20 sales data through July 3, 2020; monthly rollup based on 4-4-5 calendar.
Biosimilar WAC price used for comparing against reference product ASP until biosimilar ASP is available.
ASP: average sales price.
Source: Analysource.
Prior to the launch of Retacrit epoetin alfa biosimilar approved in May 2018 in the US [8], Epogen held the majority share of approximately 60%. Following its launch, Epogen’s share has remained stable. However, Retacrit’s share has increased up to 25% with that of the other reference, Procrit reducing from approximately 40% to 18%.
Inflammation
Anti-inflammatory biologicals make up three of top six best-selling biologicals – with Remicade (infliximab) being the third best-selling anti-inflammatory – which may lead to significant cost-saving opportunities for biosimilars in this class.
Infliximab
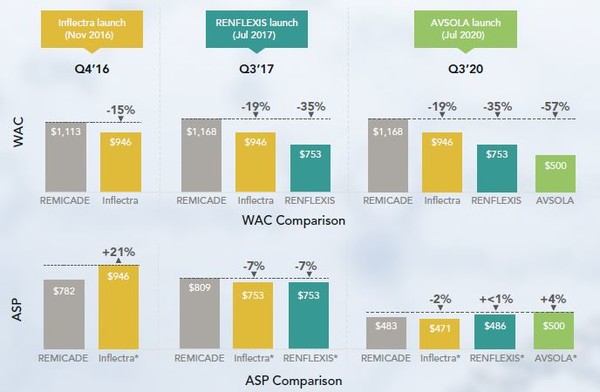
The originator infliximab product is Remicade. There have been three biosimilars launched, Inflectra, Renflexis and Avsola, each at a lower WAC than Remicade. Renflexis launched at a lower ASP than Remicade, however Inflectra and Avsola launched at a higher ASP. Further details can be seen in Figure 4.
Figure 4: WAC and ASP of infliximab biosimilars relative to reference product at launch
*ASP not available for these products at time of comparison. WAC price is used to compare with reference product ASP.
ASP: average sales price; WAC: wholesale acquisition cost.
Source: Analysource.
When it comes to the percentage change in ASP over time as compared to the originator, although Inflectra launched at a higher ASP, this soon fell below that of Remicade. The ASPs of Remicade, Inflectra and Renflexis have all reduced significantly over time. At the time that the report was compiled there was no data on Avsola.
The uptake of infliximab biosimilars has been slower than for other biosimilar products. At the time of report compilation, Inflectra and Renflexis had gained a market share of 10% by volume.
Avsola (infliximab-axxq), Inflectra (infliximab-dyyb), Ixifi (infliximab-qbtx) and Renflexis (infliximab-abda) were approved in the US in December 2019 [9], May 2018 [10], December 2017 [11] and April 2017 [12], respectively.
Related articles
The US biosimilars market in 2020
Amgen 1 Biosimilars trend report 2020
US biosimilars pipeline for supportive care, oncology and TNF inhibitors
|
LATIN AMERICAN FORUM We are pleased to announce, that starting January 2021, the launch of a new section on GaBI Online, the ‘Latin American Forum’ (in Spanish) featuring the latest news and updates on research and developments in generic and biosimilar medicines in Latin America. Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative. |
References
1. GaBI Online - Generics and Biosimilars Initiative. US biosimilars trends in oncology therapeutics [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Dec 11]. Available from: www.gabionline.net/Reports/US-biosimilars-trends-in-oncology-therapeutics
2. GaBI Online - Generics and Biosimilars Initiative. FDA approval for pegfilgrastim biosimilar Fulphila [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Dec 11]. Available from: www.gabionline.net/Biosimilars/News/FDA-approval-for-pegfilgrastim-biosimilar-Fulphila
3. GaBI Online - Generics and Biosimilars Initiative. FDA approves pegfilgrastim biosimilar Ziextenzo [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Dec 11]. Available from: www.gabionline.net/Biosimilars/News/FDA-approves-pegfilgrastim-biosimilar-Ziextenzo
4. GaBI Online - Generics and Biosimilars Initiative. FDA approves adalimumab and pegfilgrastim biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Dec 11]. Available from: www.gabionline.net/Biosimilars/News/FDA-approves-adalimumab-and-pegfilgrastim-biosimilars
5. GaBI Online - Generics and Biosimilars Initiative. FDA approves pegfilgrastim biosimilar Nyvepria [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Dec 11]. Available from: www.gabionline.net/Biosimilars/News/FDA-approves-pegfilgrastim-biosimilar-Nyvepria
6. GaBI Online - Generics and Biosimilars Initiative. FDA approves its first biosimilar [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Dec 11]. Available from: www.gabionline.net/Biosimilars/News/FDA-approves-its-first-biosimilar
7. GaBI Online - Generics and Biosimilars Initiative. FDA approves filgrastim biosimilar Nivestym [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Dec 11]. Available from: www.gabionline.net/Biosimilars/News/FDA-approves-filgrastim-biosimilar-Nivestym
8. GaBI Online - Generics and Biosimilars Initiative. FDA approves epoetin alfa biosimilar Retacrit [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Dec 11]. Available from: www.gabionline.net/Biosimilars/News/FDA-approves-epoetin-alfa-biosimilar-Retacrit
9. GaBI Online - Generics and Biosimilars Initiative. FDA approval for Amgen’s infliximab biosimilar Avsola [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Dec 11]. Available from: www.gabionline.net/Biosimilars/News/FDA-approval-for-Amgen-s-infliximab-biosimilar-Avsola
10. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in the US [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Dec 11]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-the-US
11. GaBI Online - Generics and Biosimilars Initiative. FDA approves biosimilar infliximab Ixifi [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Dec 11]. Available from: www.gabionline.net/Biosimilars/News/FDA-approves-biosimilar-infliximab-Ixifi
12. GaBI Online - Generics and Biosimilars Initiative. FDA approves biosimilar infliximab Renflexis [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Dec 11]. Available from: www.gabionline.net/Biosimilars/News/FDA-approves-biosimilar-infliximab-Renflexis
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2020 Pro Pharma Communications International. All Rights Reserved.
Source: Amgen Biosimilars
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment