A study of healthcare professionals in North America found a lack of knowledge concerning biosimilars and a need for further education on biosimilars.
US healthcare professionals need more education on biosimilars
Home/Reports
|
Posted 02/08/2013
 0
Post your comment
0
Post your comment

The study was carried out by the North American Center for Continuing Medical Education (NACCME) and surveyed more than 400 oncologists, rheumatologists, managed care professionals, pharmacists and primary care learners. The aim of the study was to understand knowledge gaps concerning biosimilars, their impact on practices and the resulting continuing educational needs for healthcare professionals.
NACCME conducted the study by distributing a brief, 5-part survey comprised of Likert scale-based and multiple-choice questions.
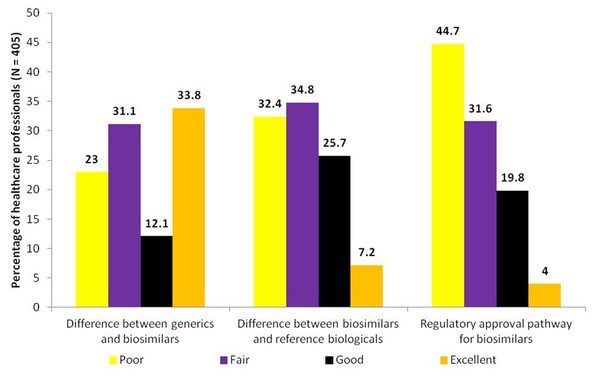
The results of the study indicated that there was a low level of understanding of the differences between biosimilars and generics, as well as of the differences between biosimilars and reference biologicals, see Figure 1. More than half of respondents (54.1%) rated their understanding of the differences between biosimilars and generics as only fair or poor. Two-thirds of respondents (67.2%) rated their understanding of the potential difference between biosimilars and reference biologicals as fair or poor. Results also indicated a low level of understanding of the regulatory approval pathway for biosimilars. The majority of respondents (76.3%) rated their understanding of the regulatory approval pathway for biosimilars as fair or poor. While most of the respondents (97%) felt that continuing education on biosimilars was at least somewhat important and three-quarters (75.8%) indicated it was important or very important to their practice.
Figure 1: US healthcare professionals understanding of biosimilars
Biosimilars are a relatively new area in the US and FDA has yet to issue finalized guidance on the subject. The results of this study indicate that comprehensive education is needed on this emerging topic in order to increase understanding amongst healthcare professionals in the US. NACCME also concluded that an in-depth discussion of biosimilars versus generics is needed, as many if not most of the surveyed healthcare professionals do not understand the distinction between generics and biosimilars and their different regulatory issues.
Related articles
EU publishes consensus report on biosimilars
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2013 Pro Pharma Communications International. All Rights Reserved.
Source: NACCME
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
Brazil and Mexico forge alliance to streamline medical approvals and boost production
EU accepts results from FDA GMP inspections for sites outside the US
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment