The Biosimilars Trend Report published in 2020 describes the landscape for biosimilars in the US. The current biosimilars available for oncology therapeutics are trastuzumab, bevacizumab and rituximab. Overall, biologicals make up approximately half of all oncology products and they are expensive due to higher development and production costs. Biosimilars offer an opportunity to reduce the cost of oncology treatments. As of July 2020, 12 oncology biosimilars were available in the US.
US biosimilars trends in oncology therapeutics
Home/Reports
|
Posted 04/12/2020
 0
Post your comment
0
Post your comment

Oncology therapeutics
In the US, oncology biosimilars have had strong growth, with trastuzumab and bevacizumab accounting for at least 40% of sales by volume.
Trastuzumab
Trastuzumab’s originator is Herceptin. This now has five competitors that were all launched at lower wholesale acquisition cost (WAC) and average sales price (ASP) than Herceptin. All trastuzumab biosimilars launched at prices of 7%‒20% less than the ASP of Herceptin. With successive launches, some launched at greater discounts than their predecessors offered but were not always launched as the cheapest trastuzumab product available, see Figure 1.
Figure 1: ASP of trastuzumab products at biosimilars launches
*Q2’20 sales data through 3 July 2020; monthly rollup based on 4-4-5 calendar.
Biosimilar WAC price used for comparing against reference product ASP until biosimilar ASP is available.
ASP: average sales price; WAC: wholesale acquisition cost.
Source: Analysource.
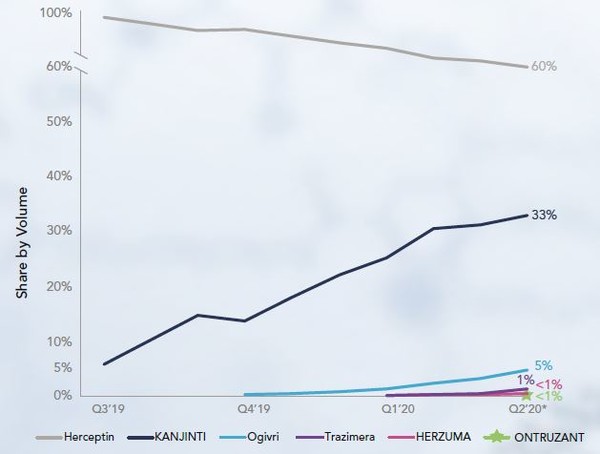
Trastuzumab biosimilars have had high uptake and at the time the report was published, Kanjinti approved in the US in June 2019 [1] had captured a 33% market share. The other biosimilars had not been on the market for long, so their share was much smaller.
Other trastuzumab biosimilars: Herzuma (trastuzumab-pkrb), Ogivri (trastuzumab-dkst), Ontruzant (trastuzumab-dttb), Trazimera (trastuzumab-qyyp) have also been approved in the US [2].
Bevacizumab
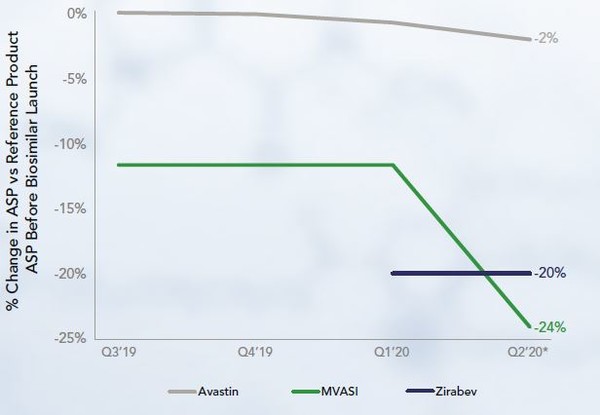
Bevacizumab’s originator is Avastin. This now has two competitors that were launched at lower WAC and ASP than Avastin. The second biosimilar to launch, Zirabev, had a lower ASP than the first, Mvasi. However now, the ASP of Mvasi is lower than that of Zirabev, and both remain lower than the ASP of Avastin, although that has decreased by 2% since biosimilar market entry, see Figure 2.
Figure 2: Biosimilar uptake curve for trastuzumab products
*Q2’20 sales data through 3 July 2020; monthly rollup based on 4-4-5 calendar.
Source: OBU Customer Data Pack Weekly (IQVIA DDD + Chargeback).
Mvasi and Zirabev were approved in the US in September 2017 [3] and June 2019 [4], respectively.
There has been a strong adoption of bevacizumab biosimilars with Mvasi capturing 38% of the market in nine months.
Rituximab
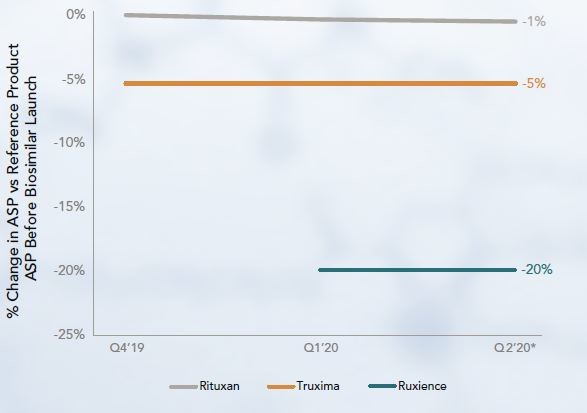
Rituximab’s originator is Rituxan. This now has two competitors that were launched at lower WAC and ASP than Rituxan. The second biosimilar to launch, Ruxience, had a lower ASP than the first, Truxima. Both biosimilars were relatively new to the market and no price concessions were yet observed for them, although Rituxan’s ASP had reduced by 1%, see Figure 3. Their future uptake levels are yet to be discovered.
Figure 3: ASP of rituximab products at biosimilars’ launches
*Q2’20 sales data through 3 July 2020; monthly rollup based on 4-4-5 calendar.
Biosimilar WAC price used for comparing against reference product ASP until biosimilar ASP is available.
ASP: average sales price; WAC: wholesale acquisition cost.
Source: Analysource.
Ruxience and Truxima were approved in the US in July 2019 [5] and November 2018 [6], respectively.
Related articles
US market trends in oncology/nephrology supportive care and inflammation biosimilars
The US biosimilars market in 2020
Clinical review of biosimilars approved in oncology
|
LATIN AMERICAN FORUM We are pleased to announce, that starting January 2021, the launch of a new section on GaBI Online, the ‘Latin American Forum’ (in Spanish) featuring the latest news and updates on research and developments in generic and biosimilar medicines in Latin America. Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative. |
References
1. GaBI Online - Generics and Biosimilars Initiative. FDA approves trastuzumab biosimilar Kanjinti [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Dec 4]. Available from: www.gabionline.net/Biosimilars/News/FDA-approves-trastuzumab-biosimilar-Kanjinti
2. GaBI Online - Generics and Biosimilars Initiative. Top developments in biosimilars during 2019 [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Dec 4]. Available from: www.gabi-journal.net/top-developments-in-biosimilars-during-2019.html
3. GaBI Online - Generics and Biosimilars Initiative. FDA approves bevacizumab biosimilar Mvasi [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Dec 4]. Available from: www.gabionline.net/Biosimilars/News/FDA-approves-bevacizumab-biosimilar-Mvasi
4. GaBI Online - Generics and Biosimilars Initiative. FDA approves bevacizumab biosimilar Zirabev [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Dec 4]. Available from: www.gabionline.net/Biosimilars/News/FDA-approves-bevacizumab-biosimilar-Zirabev
5. GaBI Online - Generics and Biosimilars Initiative. FDA approves rituximab biosimilar Ruxience [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Dec 4]. Available from: www.gabionline.net/Biosimilars/News/FDA-approves-rituximab-biosimilar-Ruxience
6. GaBI Online - Generics and Biosimilars Initiative. FDA approves first rituximab biosimilar Truxima [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Dec 4]. Available from: www.gabionline.net/Biosimilars/News/FDA-approves-first-rituximab-biosimilar-Truxima
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2020 Pro Pharma Communications International. All Rights Reserved.
Source: Amgen Biosimilars
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment