Biologicals are becoming increasingly important in the pharmaceuticals market due to their use to treat previously intractable diseases. The global biological market is worth approximately US$276 billion, and in 2018 seven of the top 10 best-selling drugs were biologicals compared with only three in 2008 [1].
US biosimilars pipeline for immunosuppressants, insulin and ophthalmology
Home/Reports
|
Posted 17/07/2020
 0
Post your comment
0
Post your comment
The biosimilar market is also growing rapidly. In Europe, the share of the European Union’s biologicals market that is subject to competition from biosimilars increased from 9% back in 2013 to 29% in 2018 [2].
However, the situation in the US is not quite so rosy. Biosimilars currently make up only 2.3% of the US biologicals marketplace. Currently, 90% of global biosimilars sales take place in Europe, despite 60% of overall biologicals sales occurring in the US [3].
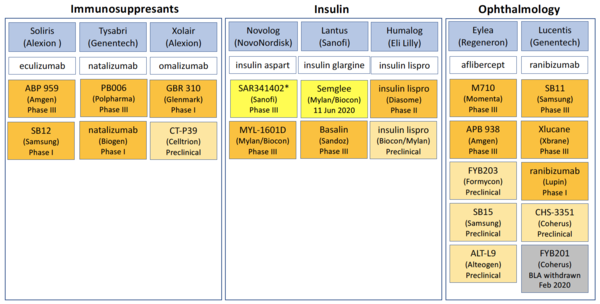
Despite the lack of uptake of biosimilars in the US, the pipeline for biosimilars still looks healthy. In fact, in the areas of immunosuppressants, insulin and ophthalmology, no fewer than 22 biosimilars are in the pipeline for the US biosimilar marketplace, see Table 1 [4].
Immunosuppressant drugs are a class of drugs that suppress, or reduce, the strength of the body's immune system. Some of these drugs are used to make the body less likely to reject a transplanted organ, such as a liver, heart, or kidney. These drugs are called anti-rejection drugs. Other immunosuppressant drugs are often used to treat autoimmune disorders such as lupus, psoriasis and rheumatoid arthritis.
Insulin biologicals are manufactured insulin (a peptide hormone produced by the pancreas). They are used in the management of type I and type II diabetes (a condition in which the body does not produce insulin and therefore cannot control the amount of sugar in the blood).
Ophthalmology biologicals include treatments for wet age-related macular degeneration (wet AMD).
Table 1: US pipeline for immunosuppressant, insulin and ophthalmology biosimilars
Related articles
US biosimilars pipeline for growth hormone and infertility drugs
US biosimilars pipeline for supportive care, oncology and TNF inhibitors
Approval and launch dates for US biosimilars
References
1. Derbyshire M, Shina S. Patent expiry dates for best-selling biologicals: 2018 update. Generics and Biosimilars Initiative Journal (GaBI Journal). 2019;8(1):24-31. doi:10.5639/gabij.2019.0801.003
2. GaBI Online - Generics and Biosimilars Initiative. Biosimilars market and opportunities in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Jul 17]. Available from: www.gabionline.net/Reports/Biosimilars-market-and-opportunities-in-Europe
3. GaBI Online - Generics and Biosimilars Initiative. The sluggish US biosimilars market [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Jul 17]. Available from: www.gabionline.net/Reports/The-sluggish-US-biosimilars-market
4. McGowan S, Jesse M. Biosimilars pipeline report. AmerisourceBergen, May 2020.
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2020 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets









Post your comment