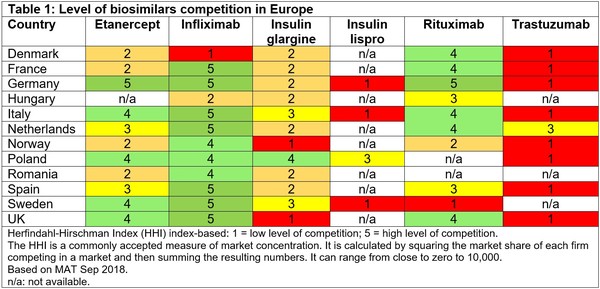
Patient use of biologicals has generally increased following the introduction of biosimilars. Despite this, levels of competition in Europe are variable across molecules and countries, see Table 1, Murray Aitken explained at the 17th Biosimilar Medicines Conference [1].
The level of biosimilars competition in Europe varies
Home/Reports
|
Posted 24/05/2019
 0
Post your comment
0
Post your comment
Infliximab has one of the highest levels of competition in Europe. The first infliximab biosimilars, Inflectra/Remsima, were approved in the European Union (EU) back in September 2013 and there are now four infliximab biosimilars approved in Europe [2].
Trastuzumab, on the other hand, has one of the lowest levels of competition in Europe. Although there are now five trastuzumab biosimilars approved in the EU, the first trastuzumab biosimilar, Ontruzant, was only approved in November 2017 [2], perhaps explaining the low penetration as yet.
Some countries, such as Poland, have also managed to achieve high levels of biosimilar penetration. Although in Poland automatic substitution at the pharmacy level is not recommended, lack of specific guidance or laws has made it possible to occur [3].
Given the huge potential savings to be made, policies to encourage the use of biosimilars are essential for the future sustainability of the biosimilars market.
Conflict of interest
The author of the presentation [1] did not provide any conflict of interest statement.
Related articles
Factors contributing to long-term sustainability of biosimilars
Biosimilars market and opportunities in Europe
Challenges for biosimilar sustainability in Europe
References
1. Aitken M. Advancing long-term biosimilar sustainability in Europe: the challenges ahead. Medicines for Europe 17th Biosimilar Medicines Conference; 28-29 March 2019; Amsterdam, The Netherlands.
2. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 May 24]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Europe
3. GaBI Online - Generics and Biosimilars Initiative. Saving money in the European healthcare system with biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 May 24]. Available from: www.gabionline.net/Biosimilars/Research/Saving-money-in-the-European-healthcare-system-with-biosimilars
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2019 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment